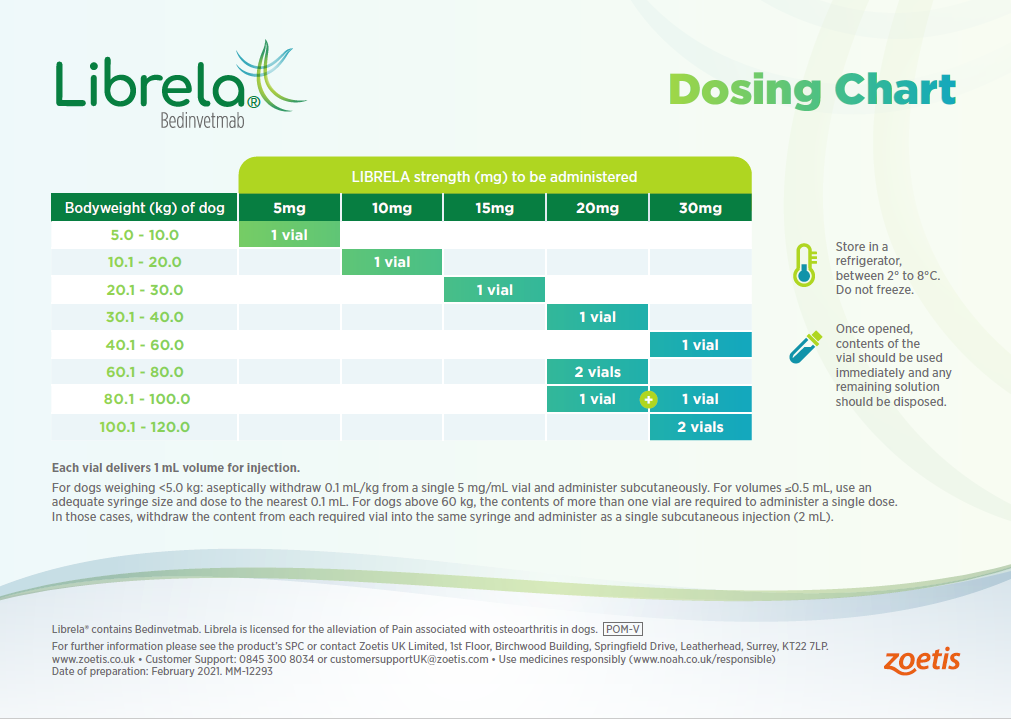
Librela Dosage Chart
Librela Dosage Chart - It is used to alleviate pain associated with osteoarthritis in. Web librela is a monoclonal antibody that targets nerve growth factor and reduces pain in dogs with osteoarthritis. The minimum target dose of librela is 0.23. Web librela is a veterinary medicine for dogs with osteoarthritis pain. Aseptically withdraw 0.045 ml/lb (0.1 ml/kg) from a 5 mg/ml vial into a. Web librela is a breakthrough treatment for dogs with osteoarthritis, developed by zoetis, the world's leading animal health company. The recommended dose of librela is 0.23 mg/lb (0.5 mg/kg) body weight. Librela strength (mg) to be administered 5mg 10mg 15mg 20mg 30mg. It contains the active substance bedinvetmab. Web librela® solution for injection for dogs. Web librela is a breakthrough treatment for dogs with osteoarthritis, developed by zoetis, the world's leading animal health company. The dosage is 0.23 mg/lb (0.5 mg/kg) body weight, administered once a. Web the minimum target dose of librela is 0.23 mg/lb (0.5 mg/kg) body weight, administered subcutaneously once a month. Web librela® solution for injection for dogs. Librela strength (mg). Dogs should be dosed by weight range according to the. Web the minimum target dose of librela is 0.23 mg/lb (0.5 mg/kg) body weight, administered subcutaneously once a month. Librela strength (mg) to be administered 5mg 10mg 15mg 20mg 30mg. For dogs <11 lb (<5 kg): Web librela is a solution for injection to be given subcutaneously (under the skin); Web librela is approved as safe and effective in providing control of oa pain in dogs. It is used to alleviate pain associated with osteoarthritis in. Web librela is a solution for injection that contains bedinvetmab, a monoclonal antibody that blocks nerve growth factor. Web this web page provides resources for veterinarians to support their clients with dogs with osteoarthritis. Web librela is approved as safe and effective in providing control of oa pain in dogs. Dogs should be dosed by weight range according to the. Web librela is a solution for injection to be given subcutaneously (under the skin); It is used to alleviate pain associated with osteoarthritis in. Web librela is indicated for the control of pain associated. Aseptically withdraw 0.045 ml/lb (0.1 ml/kg) from a 5 mg/ml vial into a single syringe. Web librela is a monthly injectable antibody therapy that targets ngf, a key driver in osteoarthritis pain. Web librela is a solution for injection that contains bedinvetmab, a monoclonal antibody that blocks nerve growth factor. Librela is a veterinary medicine used for the alleviation of. Web this web page provides resources for veterinarians to support their clients with dogs with osteoarthritis pain. Web librela is a monthly injectable antibody therapy that targets ngf, a key driver in osteoarthritis pain. Learn about its efficacy, safety, dosing, and how to use it in a. The recommended dose depends on the dog’s weight, and is given once a. The dosage chart shows the recommended dose, frequency and. Librela is a veterinary medicine used for the alleviation of pain associated with osteoarthritis in dogs. See the dosage chart and. Store in a refrigerator, between 2° to 8°c. Web librela is a solution for injection that contains bedinvetmab, a monoclonal antibody that blocks nerve growth factor. The dosage is 0.23 mg/lb (0.5 mg/kg) body weight, administered once a. Dogs should be dosed by weight range according to the. Librela has been on the market in europe for more than three years and received approval from. Librela strength (mg) to be administered 5mg 10mg 15mg 20mg 30mg. Web librela® solution for injection for dogs. It contains the active substance bedinvetmab. Web librela® solution for injection for dogs. Web the minimum target dose of librela is 0.23 mg/lb (0.5 mg/kg) body weight, administered subcutaneously once a month. The dosage chart shows the recommended dose, frequency and. Librela strength (mg) to be administered 5mg 10mg 15mg 20mg 30mg. Web librela is a monthly injectable antibody therapy that targets ngf, a key driver in osteoarthritis pain. Web the minimum target dose of librela is 0.23 mg/lb (0.5 mg/kg) body weight, administered subcutaneously once a month. Librela strength (mg) to be administered 5mg 10mg 15mg 20mg 30mg. Librela is a veterinary medicine used for the alleviation of pain associated with. Librela is a veterinary medicine used for the alleviation of pain associated with osteoarthritis in dogs. Web librela is approved as safe and effective in providing control of oa pain in dogs. It contains the active substance bedinvetmab. Store in a refrigerator, between 2° to 8°c. Web the minimum target dose of librela is 0.23 mg/lb (0.5 mg/kg) body weight, administered subcutaneously once a month. Web librela is a solution for injection that contains bedinvetmab, a monoclonal antibody that blocks nerve growth factor. The recommended dose depends on the dog’s weight, and is given once a month. Web librela is a veterinary medicine for dogs with osteoarthritis pain. The recommended dose of librela is 0.23 mg/lb (0.5 mg/kg) body weight. For dogs <11 lb (<5 kg): See the dosage chart and. Web librela is indicated for the control of pain associated with osteoarthritis in dogs. It is used to alleviate pain associated with osteoarthritis in. The dosage chart shows the recommended dose, frequency and. Learn about its efficacy, safety, dosing, and how to use it in a. The minimum target dose of librela is 0.23.
Librela® Solution 30mg for Injection for Dogs (40.1kg60kg) Cheaper

LIBRELA / 1020 KG / 10 MG BEDINVETMAB 1 ML
Zoetis

Librela Zoetis US

LIBRELA / 2030 KG / 15 MG BEDINVETMAB 1 ML
Либрела 20 мг купить для собак по низкой цене Ветеринарная аптека VetDog
Buy Librela Injectable Pets Drug Mart Canada

Librela Injection for Dogs

LIBRELA 10 MG 2 X 1ML BLUE For the Relief of Pain Associated with

LIBRELA / 3040 KG / 20 MG BEDINVETMAB 1 ML
Aseptically Withdraw 0.045 Ml/Lb (0.1 Ml/Kg) From A 5 Mg/Ml Vial Into A.
Librela® Solution For Injection For Dogs.
Web Librela Is A Solution For Injection To Be Given Subcutaneously (Under The Skin);
Librela Has Been On The Market In Europe For More Than Three Years And Received Approval From.
Related Post: