Vapour Pressure Chart
Vapour Pressure Chart - Vapor pressure of water from 0 °c to 100 °c. Yes, it sounds simple, but there are a couple of hints that speed things up. Vapourtec recommends where possible using several bprs in series rather than one large bpr. At the normal boiling point of a liquid, the vapor pressure is equal to the standard atmospheric pressure defined as 1 atmosphere, [7] 760 torr, 101.325 kpa, or 14.69595 psi. The imperial chart indicates absolute pressure. Nist chemistry webbook, srd 69. Web interactive vapor pressure deficit chart/calculator for horticulture, with dew point. Vapor pressure is directly proportional to temperature). Search search is the most efficient way to navigate the engineering toolbox. Web the following charts and table provide a comprehensive list of water vapor pressure at different temperatures under 1 atmospheric (atm) pressure. Vapor pressures of the elements (data page) notes. Cannabis, tomatoes, leafy greens, cucumber. We reworked the leader of the super shoe pack and tuned the engine underneath to help you chase personal bests from a 10k to. Web this page looks at how the equilibrium between a liquid (or a solid) and its vapor leads to the idea of a. Vapourtec recommends where possible using several bprs in series rather than one large bpr. For more information on flow chemistry systems and services please use the contact methods below. We reworked the leader of the super shoe pack and tuned the engine underneath to help you chase personal bests from a 10k to. At the normal boiling point of a. Nist chemistry webbook, srd 69. Yes, it sounds simple, but there are a couple of hints that speed things up. Imperial gauge pressure can be calculated as. The imperial chart indicates absolute pressure. Kinematic viscosity table chart liquids. Web vapor pressure (or vapour pressure) is the equilibrium pressure of a vapor above its liquid or solid state in a closed container. We reworked the leader of the super shoe pack and tuned the engine underneath to help you chase personal bests from a 10k to. Values are given in terms of temperature necessary to reach the specified pressure.. Web with this vapor pressure of water calculator, you can find the vapor pressure at a particular temperature according to five different formulas. ” crc handbook of chemistry and physics,. Web what is the fastest way to boil water? Vapourtec’s vapour pressure chart is only available to knowledge base members. Web the vapor pressure of water is the pressure exerted. (b) when sufficient molecules are in the vapor phase for a given temperature, the rate of condensation equals the. Web vapour pressure of common solvents at elevated temperatures. One hint is to put a lid on the pot. Vapor pressure is directly proportional to temperature). One of the first lessons in cooking is how to boil water. Vapourtec’s vapour pressure chart is only available to knowledge base members. Water tends to evaporate or vaporize by projecting molecules into the space above its surface. We reworked the leader of the super shoe pack and tuned the engine underneath to help you chase personal bests from a 10k to. Call us on +44 (0)1284 728659 or email us. Kinematic. Luxel corporation, friday harbor, washington. ” crc handbook of chemistry and physics,. Web fluid characteristics chart table: Valid results within the quoted ranges from most equations are included in the table for comparison. For vapour pressure kpa, density, kinematic viscosity at specified temperature. Vapor pressures of the elements (data page) notes. Luxel corporation, friday harbor, washington. Web what is the fastest way to boil water? Web the vapor pressure of propane (c3h8) depends on the temperature. It also looks at how saturated vapor pressure varies with temperature, and the relationship between saturated vapor. We reworked the leader of the super shoe pack and tuned the engine underneath to help you chase personal bests from a 10k to. Web the following charts and table provide a comprehensive list of water vapor pressure at different temperatures under 1 atmospheric (atm) pressure. One hint is to put a lid on the pot. Liquid densities fluid characteristics. Water tends to evaporate or vaporize by projecting molecules into the space above its surface. Using this 1st calculator, you insert temperature in °f, and get the vapor pressure of water in terms of kpa, psi, mmhg, bar, atm, torr. It also looks at how saturated vapor pressure varies with temperature, and the relationship between saturated vapor. Imperial gauge pressure can be calculated as. Web explore a comprehensive table of vapor pressure values for a wide range of liquids in both si (kpa) and us customary (psi) units. The imperial chart indicates absolute pressure. (1 atm = 101,325 pa) click on the icon to switch between si (kpa) and us customary (psi) units. Values are given in terms of temperature necessary to reach the specified pressure. Web fluid characteristics chart table: Temperatures (oc) at which the vapor pressures (torr) are: Web this page looks at how the equilibrium between a liquid (or a solid) and its vapor leads to the idea of a saturated vapor pressure. Web the vapor pressure of propane (c3h8) depends on the temperature. Web figure 11.4.2 vapor pressure (a) when a liquid is introduced into an evacuated chamber, molecules with sufficient kinetic energy escape from the surface and enter the vapor phase, causing the pressure in the chamber to increase. For vapour pressure kpa, density, kinematic viscosity at specified temperature. Pressure drop along pipe length. Web generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e.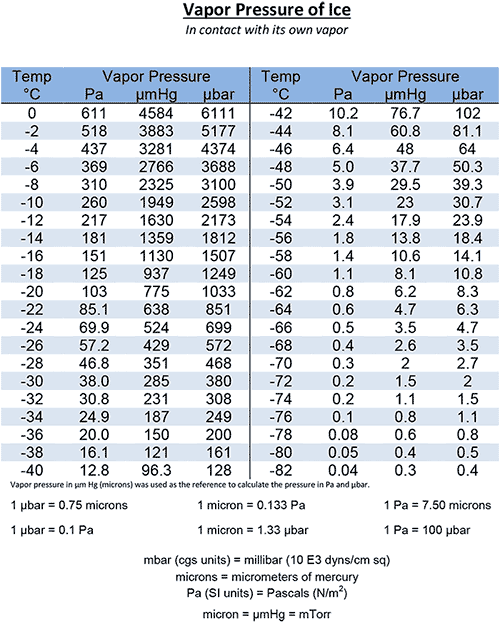
Vapor Pressure of Ice Chart

Datasheets Vapourtec

Water activity and vapour pressure values. Download Table
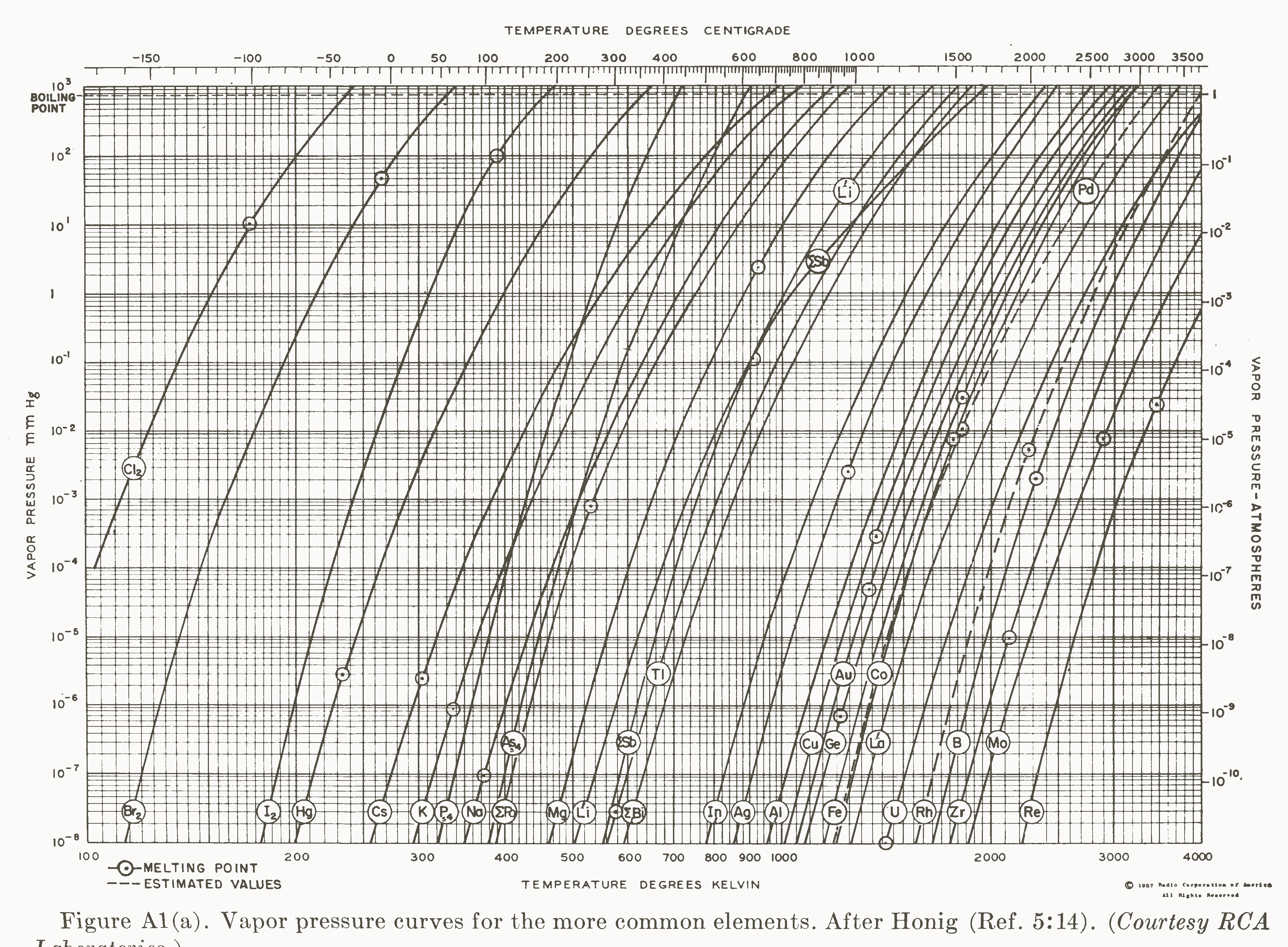
Vapor pressures of the Chemical Elements, vapor pressure of metals and

Conservation physics Fundamental microclimate concepts

Water Vapour Pressure Chart Bar

Vapour Pressure Of Water Chart
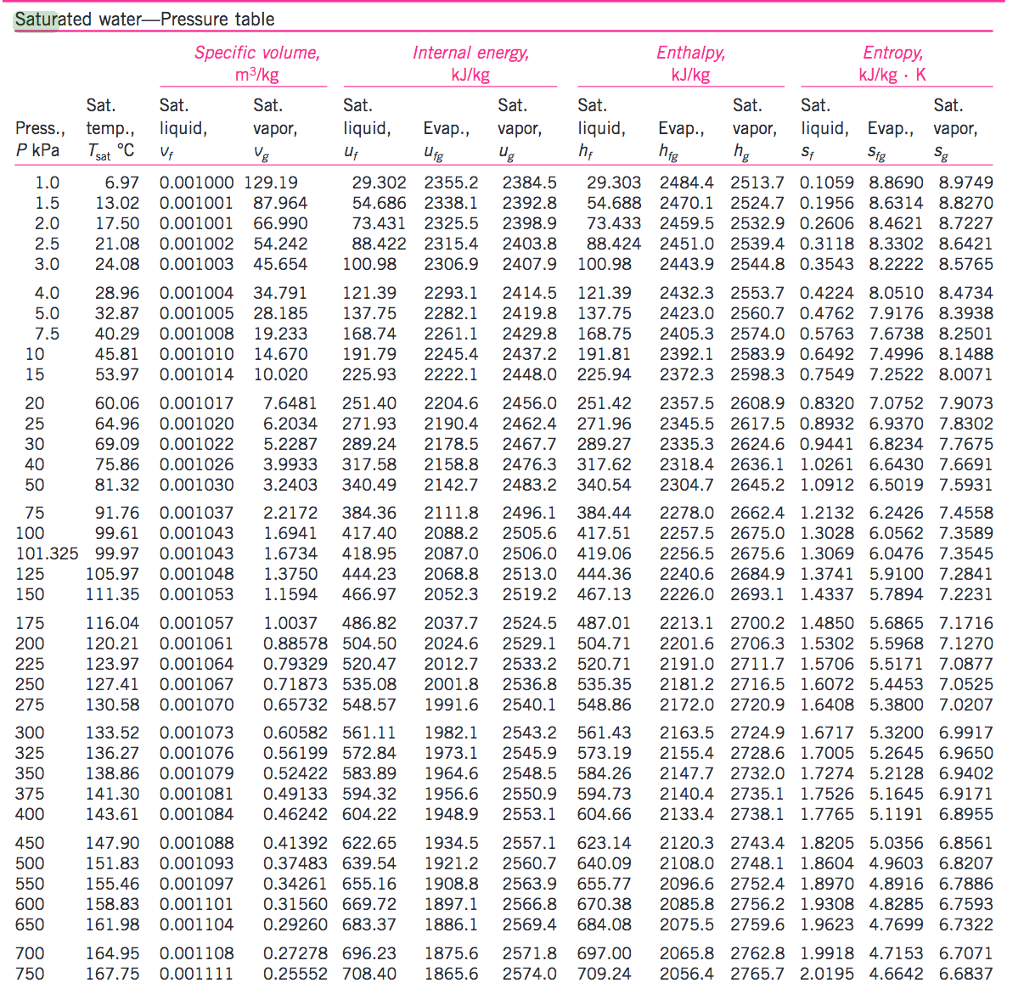
Vapor Pressure Chart For Water
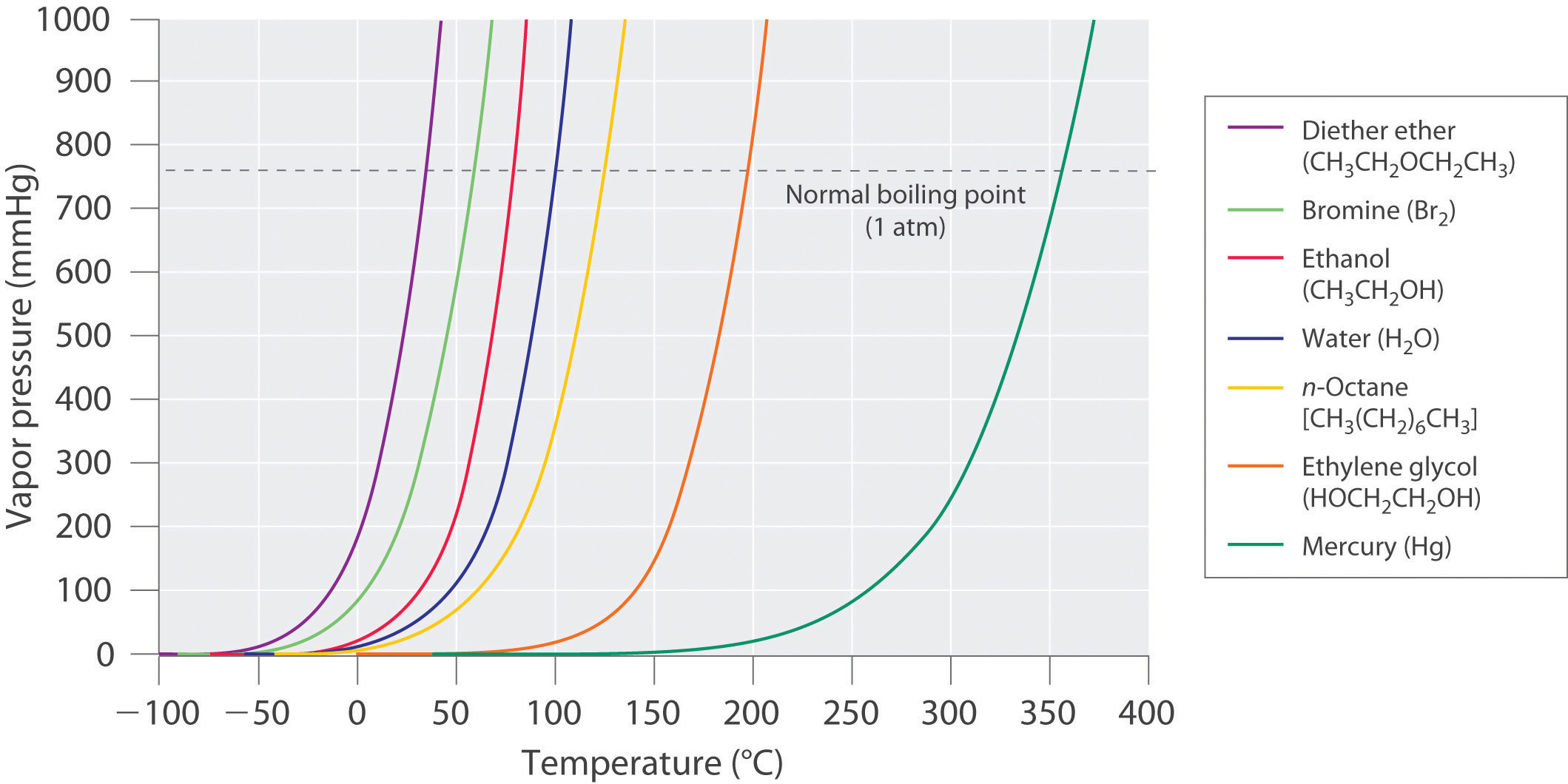
Chapter 11.4 Vapor Pressure Chemistry LibreTexts

Water Vapour Pressure Chart
Web What Is The Fastest Way To Boil Water?
Valid Results Within The Quoted Ranges From Most Equations Are Included In The Table For Comparison.
In This Type Of Closed System, Some Molecules Of A Liquid Or Solid Have Enough Kinetic Energy To Escape At The Surface And Enter The Vapor (Gas) Phase.
This Chart Shows That This Trend Is True For Various Substances With Differing Chemical Properties.
Related Post: