Vapor Pressure Chart For Water
Vapor Pressure Chart For Water - The picture above has water boiling uncovered with the steam escaping to the atmosphere. (1 atm = 101,325 pa) click on the icon to switch between si (kpa) and us customary (psi) units. The line on the graph shows the boiling temperature for water. Using this 1st calculator, you insert temperature in °f, and get the vapor pressure of water in terms of kpa, psi, mmhg, bar, atm, torr. The pressure up cancels the pressure down and boiling begins. Web so, at 1 atm of pressure, the saturated vapor pressure of water occurs at 100 ° c (212 ° f). A substance with a high vapor pressure is said to be volatile. Web yes, it sounds simple, but there are a couple of hints that speed things up. The output temperature is given as °c, °f, k and °r. Vapor pressure is directly proportional to temperature). Web vapor pressure formulation for water in range 0 to 100 °c. The line on the graph shows the boiling temperature for water. Crc handbook of chemistry and physics, 84th edition (2004). Vapor pressure is directly proportional to temperature). Vapor pressure of water is shared under a not declared license and was authored, remixed, and/or curated by libretexts. Choose the actual unit of pressure: ” crc handbook of chemistry and physics,. Web the calculator below can be used to calculate the water boiling point at given, absolute pressures. Web the graph shows how the saturated vapor pressure (svp) of water varies from 0°c to 100 °c. As the temperature of a liquid or solid increases its vapor pressure. The saturation vapor pressure is the pressure at which water vapor is in thermodynamic equilibrium with its condensed state. Web with this vapor pressure of water calculator, you can find the vapor pressure at a particular temperature according to five different formulas. Web so, at 1 atm of pressure, the saturated vapor pressure of water occurs at 100 ° c. Web vapor pressure formulation for water in range 0 to 100 °c. A substance with a high vapor pressure is said to be volatile. The pressure scale (the vertical one) is measured in kilopascals (kpa). Web to understand that the equilibrium vapor pressure of a liquid depends on the temperature and the intermolecular forces present. Web the vapor pressure of. (1 atm = 101,325 pa) click on the icon to switch between si (kpa) and us customary (psi) units. Conversely, vapor pressure decreases as the temperature decreases. Web below are some selected values of temperature and the saturated vapor pressures required to place the boiling point at those temperatures. Web vapor pressure of water (mmhg) at selected temperatures (°c) 0.. 1 atmosphere pressure is 101.325 kpa. Nist chemistry webbook, srd 69. Choose the actual unit of pressure: In other words, vapor pressure equals atmospheric pressure at a liquid’s boiling point. Web so, at 1 atm of pressure, the saturated vapor pressure of water occurs at 100 ° c (212 ° f). Web to understand that the equilibrium vapor pressure of a liquid depends on the temperature and the intermolecular forces present. Web the relationship between saturation vapor pressure and the absolute humidity is given by the ideal gas law for water vapor: The line on the graph shows the boiling temperature for water. Nist chemistry webbook, srd 69. Vapor pressure of. ” crc handbook of chemistry and physics,. Vapor pressure of h 2 o at various temperatures (celsius) modified from nebergall et. To understand that the relationship between pressure, enthalpy of vaporization, and temperature is given by. A substance with a high vapor pressure is said to be volatile. One hint is to put a lid on the pot. One hint is to put a lid on the pot. The output temperature is given as °c, °f, k and °r. The pressure scale (the vertical one) is measured in kilopascals (kpa). Web with this vapor pressure of water calculator, you can find the vapor pressure at a particular temperature according to five different formulas. Web the calculator below can. A substance with a high vapor pressure is said to be volatile. Web for example, air at sea level, and saturated with water vapor at 20 °c, has partial pressures of about 2.3 kpa of water, 78 kpa of nitrogen, 21 kpa of oxygen and 0.9 kpa of argon, totaling 102.2 kpa, making the basis for standard atmospheric pressure. Vapor. Web yes, it sounds simple, but there are a couple of hints that speed things up. One hint is to put a lid on the pot. Thus, at about 90 °c, the vapor pressure of water will equal the atmospheric. The pressure up cancels the pressure down and boiling begins. As the temperature of a liquid or solid increases its vapor pressure also increases. ” crc handbook of chemistry and physics,. Mbara psia mm hg in hg. Web vapor pressure formulation for water in range 0 to 100 °c. The line on the graph shows the boiling temperature for water. Web the graph of the vapor pressure of water versus temperature in figure \(\pageindex{3}\) indicates that the vapor pressure of water is 68 kpa at about 90 °c. To understand that the relationship between pressure, enthalpy of vaporization, and temperature is given by. Web generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. Conversely, vapor pressure decreases as the temperature decreases. 1 atmosphere pressure is 101.325 kpa. Published in journal of research of the… 1 september 1976. Web from crc handbook of chemistry and physics, 65th edition (rounded to two decimal places) temp, °c.![[PDF] Vapor Pressure Formulation for Water in Range 0 to 100 °C. A](https://d3i71xaburhd42.cloudfront.net/471011a1b864be3f78720f4d8ba4f21385acd117/5-Table4-1.png)
[PDF] Vapor Pressure Formulation for Water in Range 0 to 100 °C. A

Water Vapour Pressure Chart Bar
Vapor Pressure Chart For Water

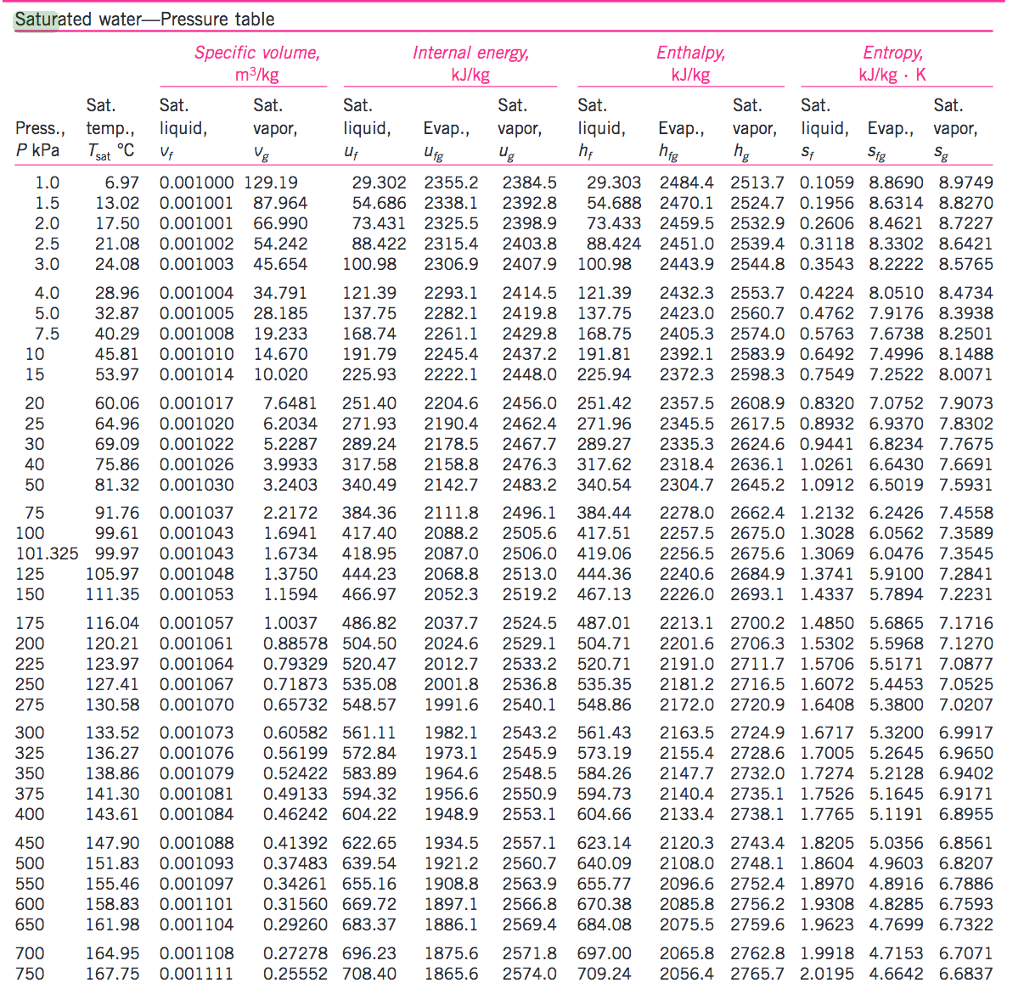
Conservation physics Fundamental microclimate concepts
![[PDF] Vapor Pressure Formulation for Water in Range 0 to 100 °C. A](https://d3i71xaburhd42.cloudfront.net/471011a1b864be3f78720f4d8ba4f21385acd117/5-Table3-1.png)
[PDF] Vapor Pressure Formulation for Water in Range 0 to 100 °C. A

Water Vapour Pressure Chart
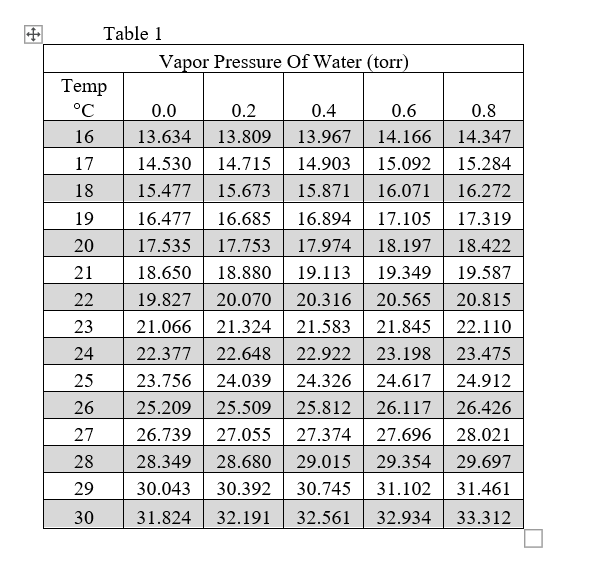
Vapour Pressure Of Water Chart

vapor pressure of water table
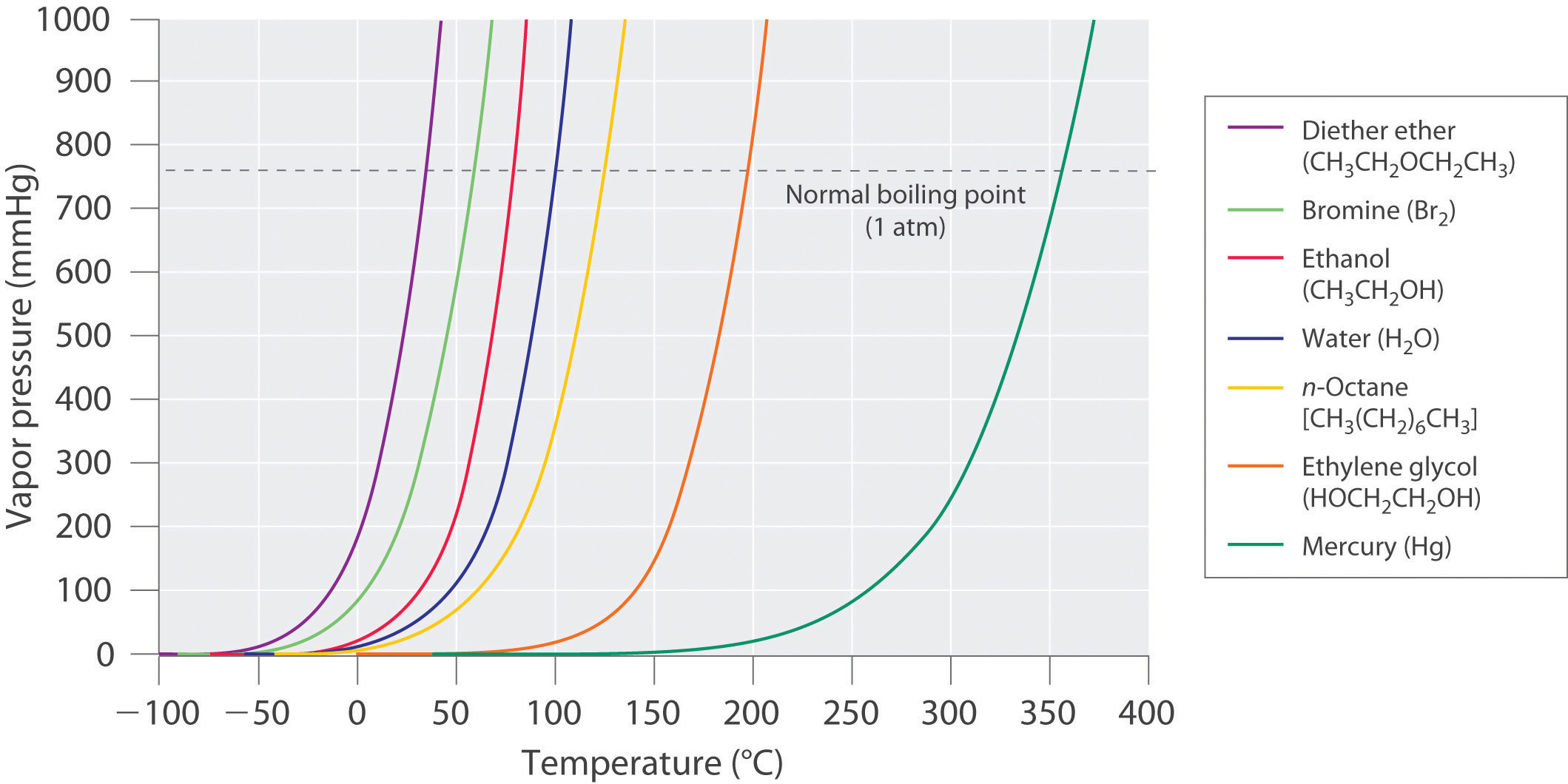
Chapter 11.4 Vapor Pressure Chemistry LibreTexts
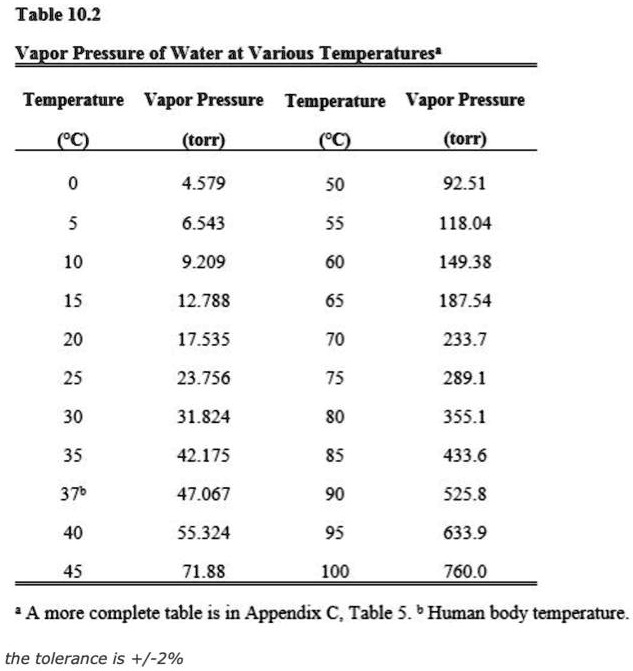
SOLVED Table 10.2 Vapor Pressure of Water at Various Temperatures
Web So, At 1 Atm Of Pressure, The Saturated Vapor Pressure Of Water Occurs At 100 ° C (212 ° F).
Web Below Are Some Selected Values Of Temperature And The Saturated Vapor Pressures Required To Place The Boiling Point At Those Temperatures.
Vapor Pressure Of H 2 O At Various Temperatures (Celsius) Modified From Nebergall Et.
The Output Temperature Is Given As °C, °F, K And °R.
Related Post: