Thermodynamic Favorability Chart
Thermodynamic Favorability Chart - Web the temperature conditions under which a process is thermodynamically favored (δg° is negative) can be predicted from the signs of δh° and δs°. At higher temperatures, the second term will become negative enough to overcome the δh resulting in a negative δ go. 2 kclo a 3 ( s) → 2 kcl ( s) + 3 o a 2 ( g) for the reaction represented above, the value of δ g ° 298 is − 226 kj/mol r x n. If δs universe is negative, then the reaction is not thermodynamically favored. Thermodynamic favorability means a reaction is spontaneous, or the reaction does not require energy to occur. When δg° < 0, the process is thermodynamically favored. Introduction to gibbs free energy. Web thermodynamic favorability refers to the intrinsic propensity of a reaction to occur spontaneously within a closed system, without requiring any external intervention. We predict that highly exothermic processes ( δh ≪ 0) that increase the entropy of a system ( δssys ≫ 0) would therefore occur spontaneously. Increases in entropy (s) are also thermodynamically favorable. Web the standard free energy of formation of a substance is the free energy change that occurs when 1 mole of the substance is formed from its constituent elements in their standard states. If the change is negative, the reaction is thermodynamically favorable and will occur spontaneously. 2 kclo a 3 ( s) → 2 kcl ( s) + 3. Web the temperature conditions under which a process is thermodynamically favored (δg° is negative) can be predicted from the signs of δh° and δs°. Web the standard gibbs free energy change, δg°, indicates the thermodynamic favorability of a physical or chemical process. Web gibbs free energy and thermodynamic favorability (practice) | khan academy. Web when δh° and δs° for a. Web increases in entropy (s) are also thermodynamically favorable. Web this form of the equation provides a useful link between these two essential thermodynamic properties, and it can be used to derive equilibrium constants from standard free energy changes and vice versa. In this video, we'll determine the thermodynamic favorability of a reaction with δh° < 0 and δs° <. At higher temperatures, the second term will become negative enough to overcome the δh resulting in a negative δ go. At low temperatures, δ go is positive and the reaction is thermodynamically favored. If the t δ s term is less than δ. The standard free energy of formation of kcl ( s) at 298 k is − 409 kj/mol.. We predict that highly exothermic processes ( δh ≪ 0) that increase the entropy of a system ( δssys ≫ 0) would therefore occur spontaneously. At higher temperatures, the second term will become negative enough to overcome the δh resulting in a negative δ go. Web h 2 o ( l) → h 2 o ( g) δ h o. Web the thermodynamic favorability of reactions is controlled by changes in entropy and enthalpy, and the temperature at which the reaction takes place. The position of equilibrium and therefore the electrode potential depends on factors such as: Web thermodynamic favorability refers to the intrinsic propensity of a reaction to occur spontaneously within a closed system, without requiring any external intervention.. We predict that highly exothermic processes ( δh ≪ 0) that increase the entropy of a system ( δssys ≫ 0) would therefore occur spontaneously. Web increases in entropy (s) are also thermodynamically favorable. Explain how reaction conditions can determine the product ratio in a reaction in which there is competition between thermodynamic and kinetic control. For a given process,. Cell potential & thermodynamic favorability. Coli, the use of thermodynamically more favorable pathways may improve the efficiency of pathway protein use. Thermodynamic favorability means a reaction is spontaneous, or the reaction does not require energy to occur. The position of equilibrium and therefore the electrode potential depends on factors such as: Delta s is the entropy of a. Web thermodynamically favourable means from high energy to low energy, or, put another way, from less stable to more stable. Thermodynamic favorability means a reaction is spontaneous, or the reaction does not require energy to occur. Explain how reaction conditions can determine the product ratio in a reaction in which there is competition between thermodynamic and kinetic control. The general. Web the temperature conditions under which a process is thermodynamically favored (δg° is negative) can be predicted from the signs of δh° and δs°. Web gibbs free energy and thermodynamic favorability (practice) | khan academy. Introduction to gibbs free energy. For any chemical reaction, the standard free energy change is the sum of. Web the relationship shown in equation 13.7.7. A pure element in its standard state has a standard free energy of formation of zero. If δs universe is negative, then the reaction is not thermodynamically favored. At low temperatures, δ go is positive and the reaction is thermodynamically favored. When δg° < 0, the process is thermodynamically favored. For a given process, the value of δg° can be calculated directly from. Web the thermodynamic favorability of reactions is controlled by changes in entropy and enthalpy, and the temperature at which the reaction takes place. If the t δ s term is less than δ. The standard free energy of formation of kcl ( s) at 298 k is − 409 kj/mol. Introduction to gibbs free energy. When δh° > 0 and δs° < 0, the process is. Web when δh° and δs° for a reaction have the same sign, the thermodynamic favorability of the reaction depends on temperature. Understanding the relative stability of molecules can be important for predicting relative reactivity of starting materials and the relative yields of potential products. Gibbs free energy and thermodynamic favorability. Web thermodynamic favorability refers to the intrinsic propensity of a reaction to occur spontaneously within a closed system, without requiring any external intervention. Web the temperature conditions under which a process is thermodynamically favored (δg° is negative) can be predicted from the signs of δh° and δs°. Web the relationship shown in equation 13.7.7 allows us to predict spontaneity by focusing exclusively on the thermodynamic properties and temperature of the system.
Table of Thermodynamic Values

Predicting thermodynamic favorability using energy diagrams YouTube

You should note that at low temperature, enthalpy is dominant, while at

AP Chemistry Video 92 Thermodynamic Favorability and Equilibrium

Thermodynamic Favorability

An illustrative example to show differences in ranking between

AP Chemistry 9.3 Gibbs Free Energy and Thermodynamic Favorability
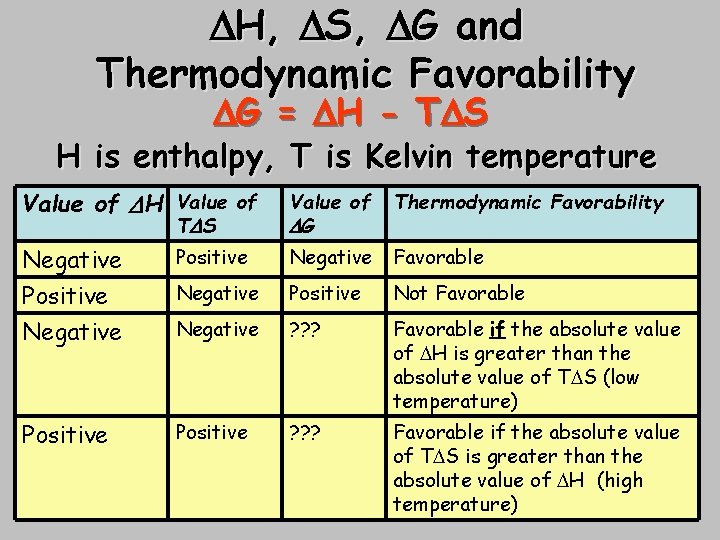
Enthalpy Entropy Free Energy and Spontaneity Thermodynamic Favorability

Thermodynamic favorability and pathway yield as evolutionary tradeoffs

Thermodynamic favorability and pathway yield as evolutionary tradeoffs
Delta S Is The Entropy Of A.
This Condition Describes An Endothermic Process That Involves An Increase In System Entropy.
The General Rule Is That If The Entropy Of The Universe (Thermodynamic System Plus Its Surroundings) Is Positive, Then The Reaction Is Thermodynamically Favored.
Increases In Entropy (S) Are Also Thermodynamically Favorable.
Related Post: