Salt Brine Chart
Salt Brine Chart - Making brine at the correct concentration is critical in the snow and ice industry. Web in diverse contexts, brine may refer to the salt solutions ranging from about 3.5% (a typical concentration of seawater, on the lower end of that of solutions used for brining foods) up to about 26% (a typical saturated solution, depending on temperature). How to make a brine? Morton can help you learn how to brine so your next dish can be tasty, tender and juicy. *** transition temperature from anhydrous salt to dihydrate. Measurement of brine solution is critical. How much salt to add? 373 k), saturated sodium chloride brine is about 28% salt by weight. You can up the flavor profile by adding spices like bay leaves, peppercorns, citrus, and even flavors like apple juice. Accurate fermenting brine for big quantities. It’s a “total salt concentration chart.” what? You can up the flavor profile by adding spices like bay leaves, peppercorns, citrus, and even flavors like apple juice. Web are you looking for a brine chart? Morton can help you learn how to brine so your next dish can be tasty, tender and juicy. How much salt to add? Choose the best water to make a brine. Web use the brine calculator to determine the accurate salt to water ratio and make a perfect brine for fermenting vegetables. Measurement of brine solution is critical. It’s a “total salt concentration chart.” what? Determine the amount of salt required to make a specified salt brine, e.g. Web for more information or to purchase a salometer (astm e 100, brine tester), visit: Choose the best water to make a brine. What is a brine for fermentation? Determine the amount of salt required to make a specified salt brine, e.g. While simple on the surface, it’s vital that you get the exact concentration of salt amount to water. Web the most basic formula is 1 cup of salt for each gallon of water. Web use a brine calculator to get the exact salt amount needed to cover a specific area. At 100 °c (212 °f; How much salt to add? *** transition temperature from anhydrous salt to dihydrate. A 70°s brine, for example, is 70% of fully saturated brine, which at 60°f would contain 1.75lbs of nacl/gallon with a specific gravity of 1.139. While simple on the surface, it’s vital that you get the exact concentration of salt amount to water. Web the most basic formula is 1 cup of salt for each gallon of water. At 0. ‘make a seven percent brine’), which refers to the ratio of salt to water. 373 k), saturated sodium chloride brine is about 28% salt by weight. Web take the weight of the water or vegetable matter (if you’re sweating vegetables like cabbage) and multiply by the % salinity you’d like. At 0 °c (32 °f; At 100 °c (212 °f; Web for more information or to purchase a salometer (astm e 100, brine tester), visit: It’s a “total salt concentration chart.” what? Accurate fermenting brine for big quantities. Why do i need salt to ferment vegetables? The calculator enables you to accurately make a brine solution using the correct proportions of salt and water. Web graphs of the concentration of salt dissolved in water for brines. Web use the brine calculator to determine the accurate salt to water ratio and make a perfect brine for fermenting vegetables. Web use a brine calculator to get the exact salt amount needed to cover a specific area. Web tired of your turkey turning out dry? Web in. Web how to do a dry salting? Web sodium chloride brine tables for 60° f (15.5° c) brines stronger than eutectic deposit excess sodium chloride as dihydrate when cooled, and freeze at eutectic. Web tired of your turkey turning out dry? All it takes is a mixture of salt, water and spices. Web are you looking for a brine chart? Web the brine calculator calculates the amount of salt and water needed to prepare a perfect brine for fermenting vegetables. Having a weaker or stronger concentration leads to higher freezing points. Web sodium chloride brine tables for 60° f (15.5° c) brines stronger than eutectic deposit excess sodium chloride as dihydrate when cooled, and freeze at eutectic. Web the most. Determine the amount of salt required to make a specified salt brine, e.g. Web take the weight of the water or vegetable matter (if you’re sweating vegetables like cabbage) and multiply by the % salinity you’d like. Web sodium chloride brine tables for 60° f (15.5° c) brines stronger than eutectic deposit excess sodium chloride as dihydrate when cooled, and freeze at eutectic. How to make a brine. You need 1 cup of brine to cover your vegetables and you want a 2% salinity. 273 k), brine can only hold about 26% salt. Web for brine tested at a warmer or colder temperature than 60°f see table of salometer corrections * temperature at which freezing begins, ice forms, brine concentrates, and the feezing point lowers to eutectic Choose the best water to make a brine. First of all, salt neutralizes pathogenic microorganisms. Web brining is the process of submerging a cut of meat in a brine solution, which at its most basic is simply salt dissolved in water. Web by gbc kitchen. Measurement of brine solution is critical. All it takes is a mixture of salt, water and spices. A brine can also be seasoned with dried herbs and spices. Amcmudcom 2 table of contents maximum densities 2 sodium chloride, nacl 3 potassium chloride, kc 4 calcium chloride, cacl 2 5 magnesium chloride, mgcl 2 6 potassium sulfate, k 2 so 4 7 sodium formate, nacooh 8 maximum densities potassium acetate, kc. 2 is the pronounced density of calcium chloride brine relative to all.
Materials Science and Storage Salt Smart Collaborative

FS 002 The Science Behind Brining Four Part Video Lecture Stella
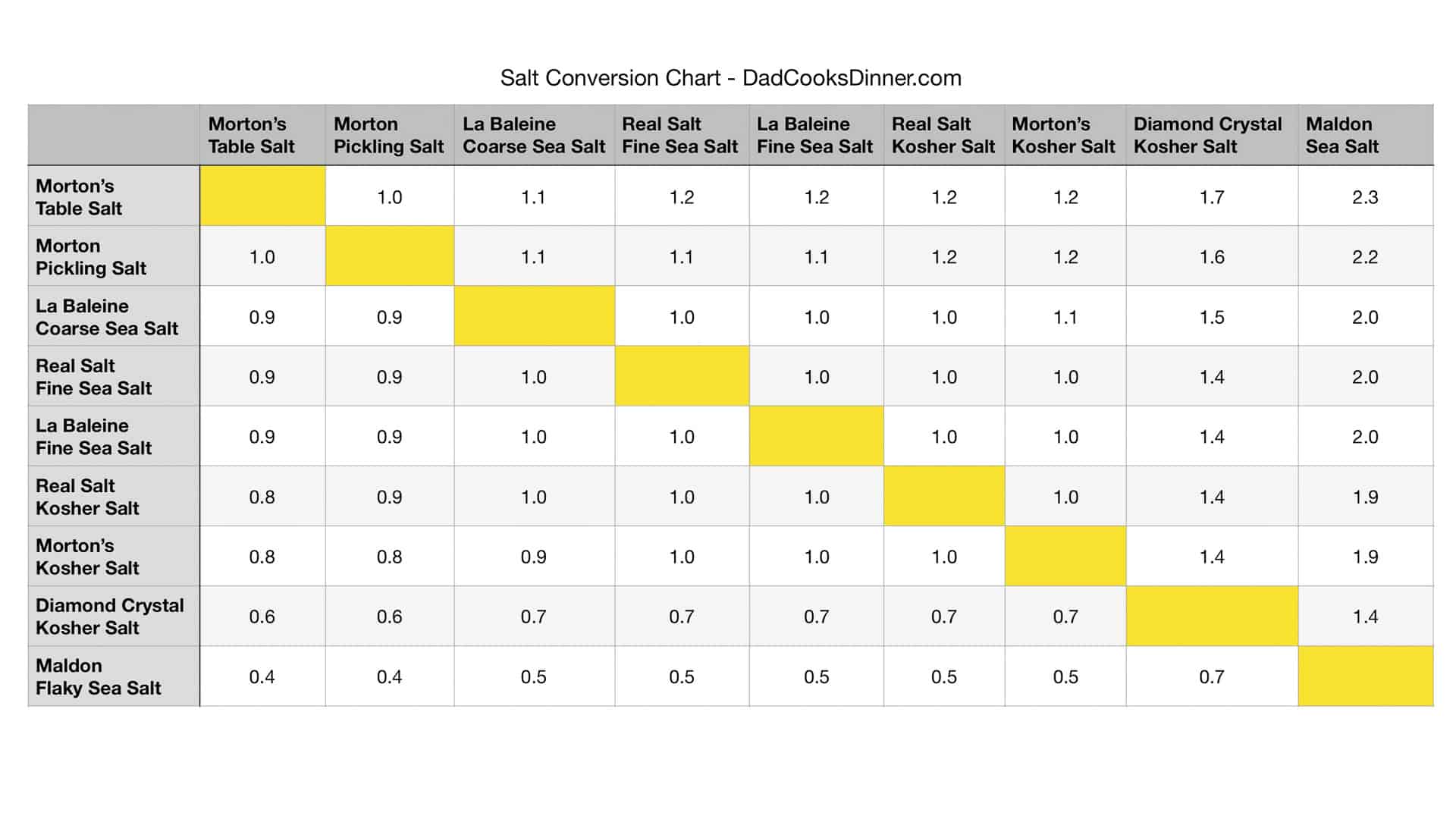
Table Salt Vs Kosher For Brining Meat Dosestream
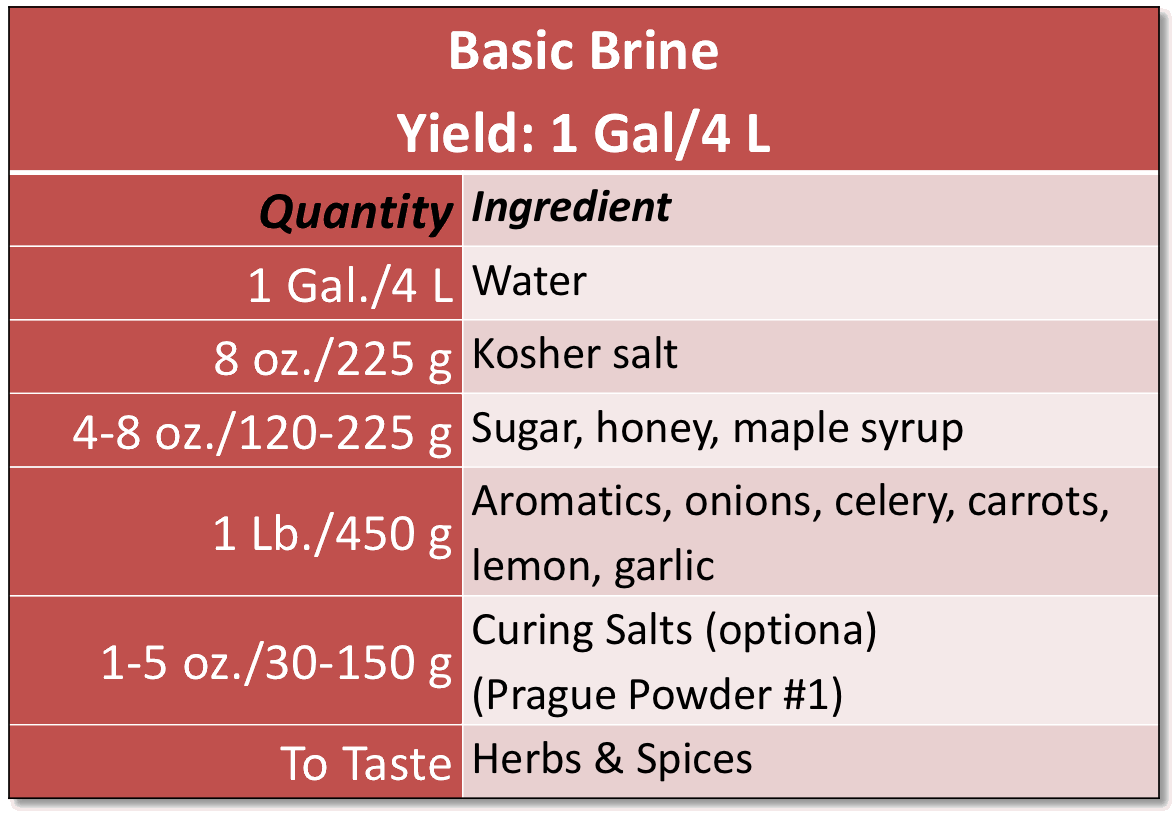
Table Salt Vs Kosher For Brining Meat Dosage Chart By Weight

Brine 101 Brine Masters

If you’re opting for a wet brine, make sure to use the right amount of

Fermentation Brine Chart
Brine Freezing Chart Sodium Chloride Salinity

Brine Chart

Brine Chart
Can We Do A Fermentation Without Salt?
Accurate Fermenting Brine For Big Quantities.
At 20 °C One Liter Of Water Can Dissolve About 357 Grams Of Salt, A Concentration Of 26.3%.
Having A Weaker Or Stronger Concentration Leads To Higher Freezing Points.
Related Post:
