Quantum Numbers Chart
Quantum Numbers Chart - Here’s a brief overview of how these numbers relate to atomic orbitals: Quantum numbers are closely related to eigenvalues of observables. Web here is the chart of allowed quantum numbers. A spherical orbital has only one orientation in space. Web the four quantum numbers used to describe atomic orbitals are the principal quantum number (n), the azimuthal quantum number (l), the magnetic quantum number (ml), and the spin quantum number (ms). This chart describes quantum numbers and gives an example. A kind of coordinate system). Explains that only two electrons are allowed per orbital, and gives shortcuts for calculating number of orbitals and total number of electrons for a given n. They represent the position, movement, and energy of the fundamental particle. Web a set of the four quantum numbers describes the unique properties of one specific electron in an atom. Web calculates number of orbitals and number of electrons in different kinds of orbitals for n = 1 to 4. Principal quantum number (n) this quantum number relates to expressing the size of the shell of an electron within an atom. Quantum numbers are closely related to eigenvalues of observables. No other ticket can have the same four parts to. Quantum numbers are closely related to eigenvalues of observables. Once again, we see how physics makes discoveries which enable other fields to grow. Web the four quantum numbers used to describe atomic orbitals are the principal quantum number (n), the azimuthal quantum number (l), the magnetic quantum number (ml), and the spin quantum number (ms). Web in atoms, there are. Web there are four quantum numbers, namely, principal, azimuthal, magnetic and spin quantum numbers. Web the four quantum numbers used to describe atomic orbitals are the principal quantum number (n), the azimuthal quantum number (l), the magnetic quantum number (ml), and the spin quantum number (ms). Just like we have four ways of defining the location of a building (country,. And the magnetic quantum number specifies orientation of the orbital in space, as can be seen in figure \(\pageindex{3}\). A spherical orbital has only one orientation in space. The principal quantum number, \(n\), describes the energy of an electron and the most probable distance of the electron from the nucleus. The only information that was important was the size of. Denoted by the symbol ‘n.’. The principal quantum number, \(n\), describes the energy of an electron and the most probable distance of the electron from the nucleus. Web there are four quantum numbers; The magnetic quantum number is not the strength of the electron’s magnetic field. Web can you guess how many people are in this stadium? Web quantum numbers chart. Web can you guess how many people are in this stadium? Web in atoms, there are a total of four quantum numbers: Web this section covers some of the more important quantum numbers and rules—all of which apply in chemistry, material science, and far beyond the realm of atomic physics, where they were first discovered. The. This chart describes quantum numbers and gives an example. Explains that only two electrons are allowed per orbital, and gives shortcuts for calculating number of orbitals and total number of electrons for a given n. We can easily see the below combinations are not possible for quantum numbers. The principal quantum number, \(n\), describes the energy of an electron and. Web a quantum number describes a specific aspect of an electron. Web the principal quantum number defines the general value of the electronic energy. No other ticket can have the same four parts to it. Once again, we see how physics makes discoveries which enable other fields to grow. The principal quantum number (n), the orbital angular momentum quantum number. Notice in the chart above, if n =3, the largest l value possible is 2, which corresponds to a d orbital. This chart describes quantum numbers and gives an example. Web can you guess how many people are in this stadium? Web the principal quantum number defines the general value of the electronic energy. Once again, we see how physics. Web a set of the four quantum numbers describes the unique properties of one specific electron in an atom. Explains that only two electrons are allowed per orbital, and gives shortcuts for calculating number of orbitals and total number of electrons for a given n. Web in quantum physics and chemistry, quantum numbers are quantities that characterize the possible states. Quantum numbers are closely related to eigenvalues of observables. Web quantum numbers for all the elements in the periodic table. The pauli exclusion principle tells us that no two electrons can share the exact same set of quantum numbers. Every electron localized in an atom can be described by four quantum numbers. Here’s a brief overview of how these numbers relate to atomic orbitals: The principle quantum number, n, represents the energy level of the electron, much like the n used in the bohr. The only information that was important was the size of the orbit, which was described by the n quantum number. Every electron in an atom has a specific, unique set of these four quantum numbers. The principal quantum number, \(n\), describes the energy of an electron and the most probable distance of the electron from the nucleus. We can easily see the below combinations are not possible for quantum numbers. Once again, we see how physics makes discoveries which enable other fields to grow. And the magnetic quantum number specifies orientation of the orbital in space, as can be seen in figure \(\pageindex{3}\). Web the principal quantum number defines the general value of the electronic energy. Web in quantum physics and chemistry, quantum numbers are quantities that characterize the possible states of the system. Denoted by the symbol ‘n.’. This chart describes quantum numbers and gives an example.
Quantum Number Definition Types Chart And Quiz

What Does Spin Quantum Number Determine DERIFIT

UPSC Preparation Quantum numbers YouTube

Four Quantum Numbers Principal, Angular Momentum, & Spin
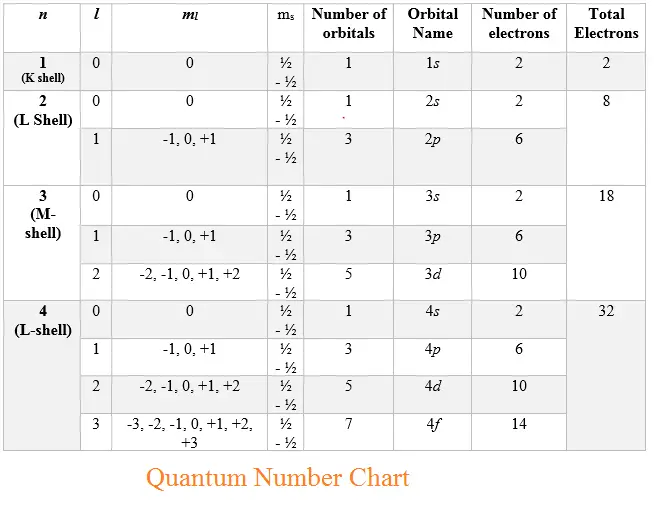
Quantum Numbers Chart physicscatalyst's Blog
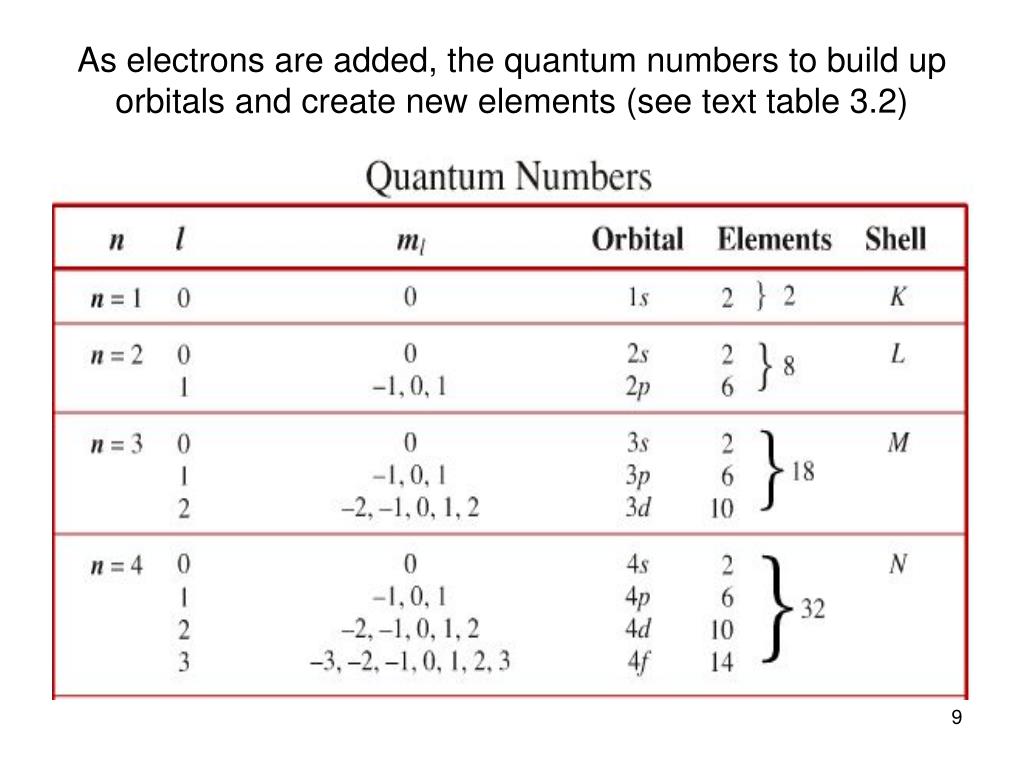
PPT Electron Configurations & Quantum Numbers PowerPoint Presentation
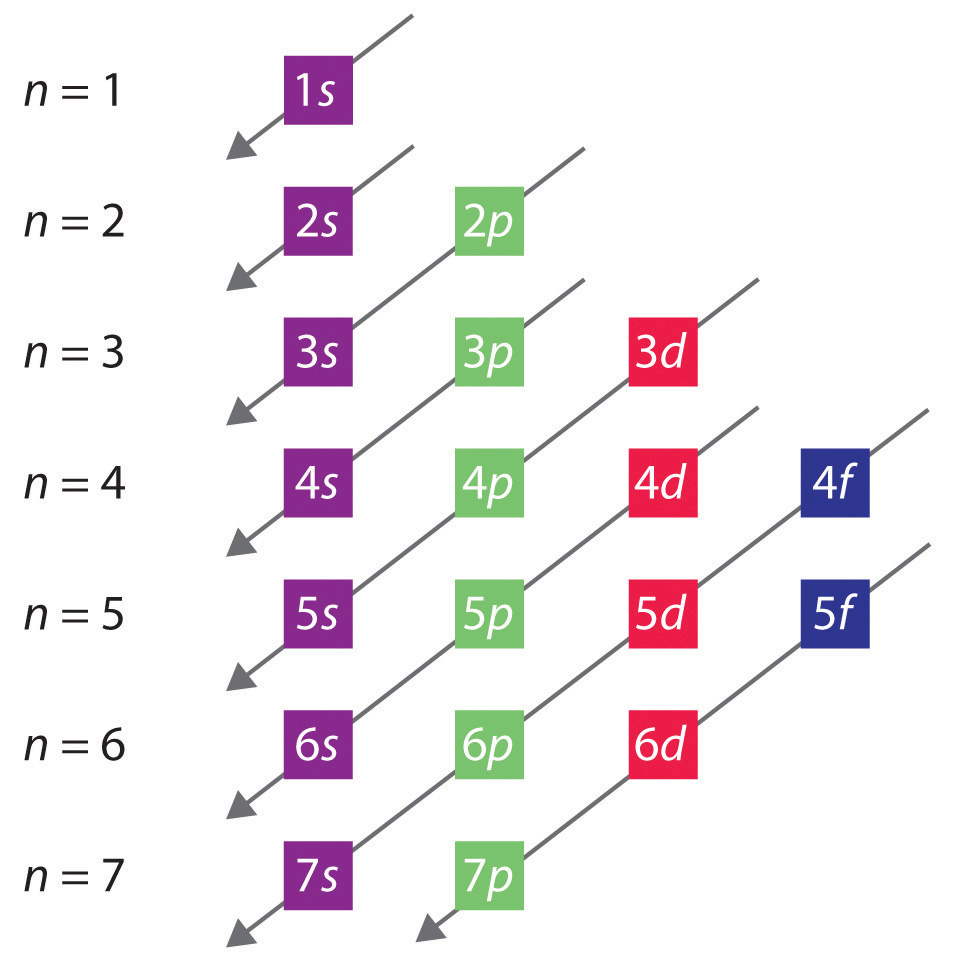
7.9 Electron Spin (A Fourth Quantum Number) Chemwiki
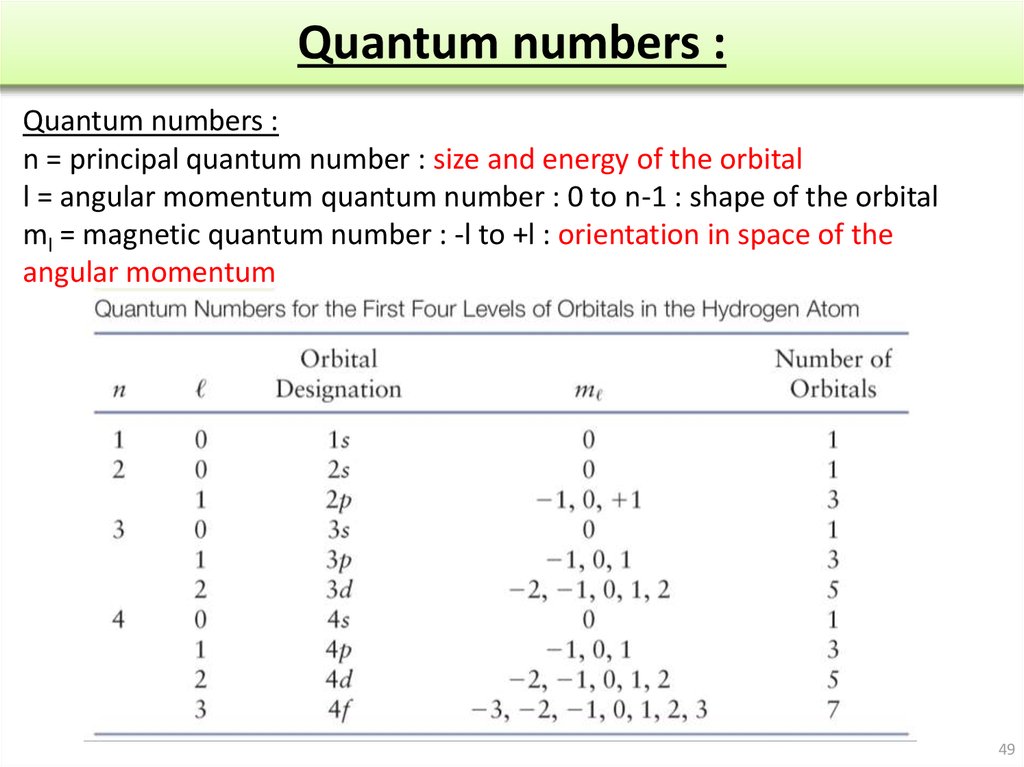
Atomic structure and properties. (Chapter 3) online presentation
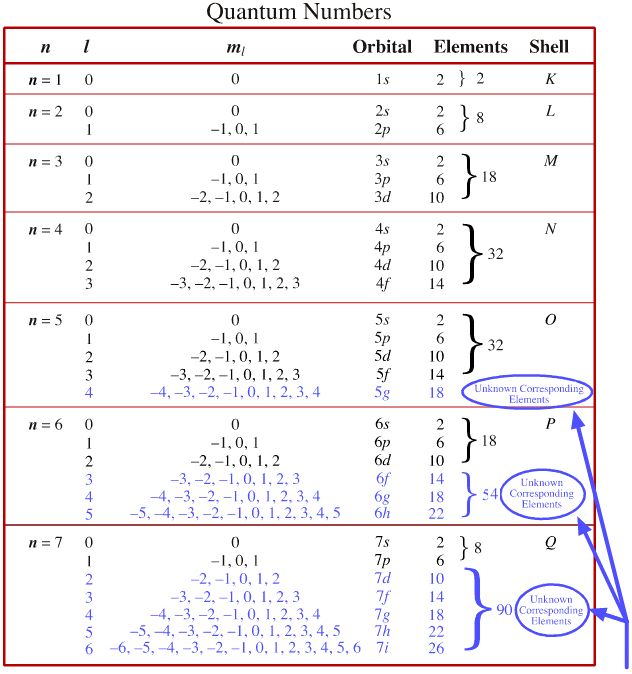
*CHEMISTRY MATRICULATION* QUANTUM NUMBERS
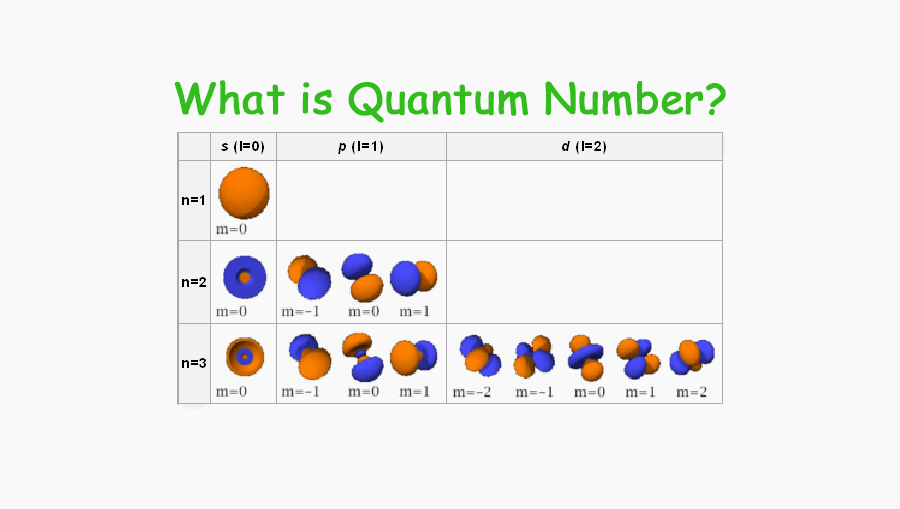
What is quantum number what do quantum number determine Tuition Tube
The Values Of The Conserved Quantities Of A Quantum System Are Given By Quantum Numbers.
Web There Are A Total Of Four Quantum Numbers:
Web A Set Of The Four Quantum Numbers Describes The Unique Properties Of One Specific Electron In An Atom.
Since Each Set Is Unique, They Serve As A Way Of Uniquely Naming Individual Electrons (I.e.
Related Post: