Ph To Poh Chart
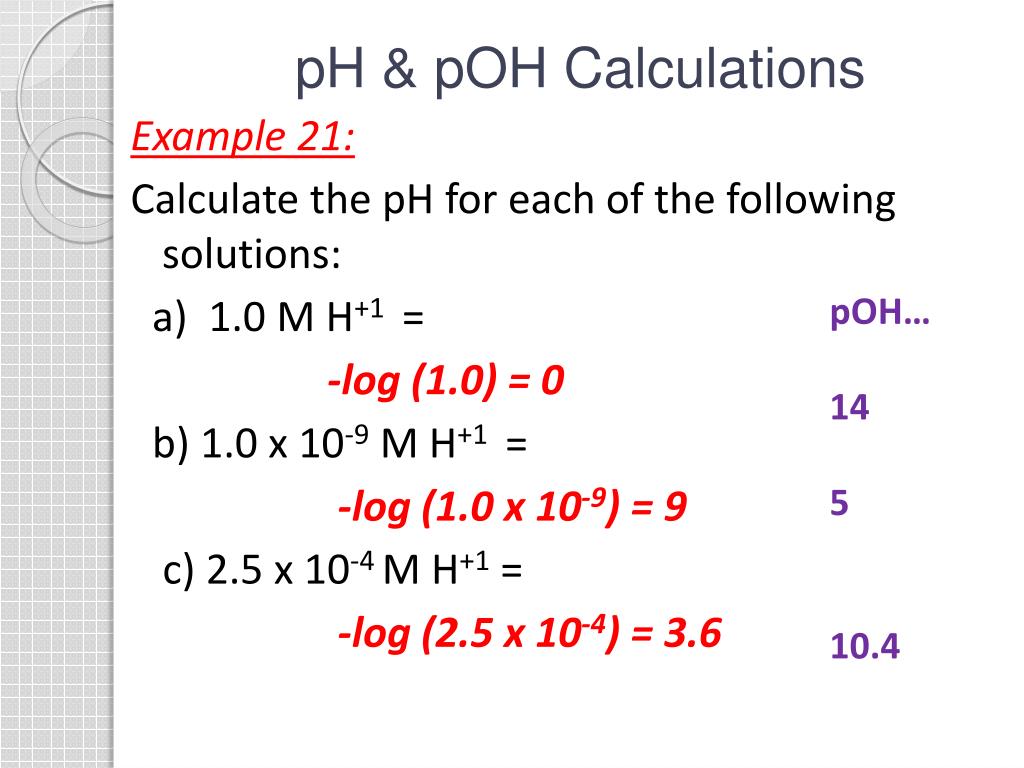
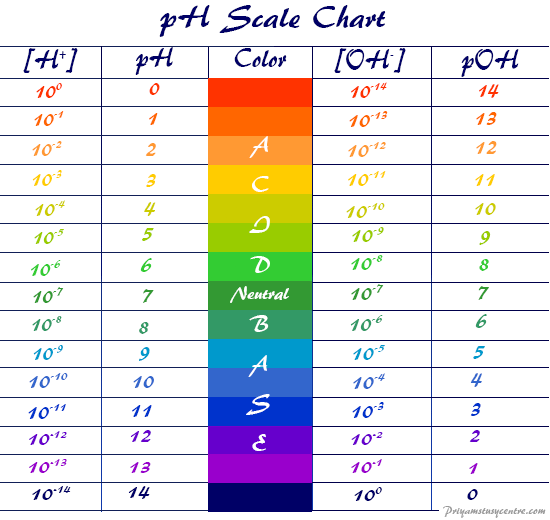
Ph To Poh Chart - It can convert ph to h +, as well as calculate ph from the ionization constant and concentration. (they are both h + ion, or proton, donors.) but they differ in the extent to which they donate h + ions to water. This is known as the ph p h scale. You can use ph to make a quick determination whether a given aqueous solution is acidic, basic, or neutral. Express hydronium and hydroxide ion concentrations on the ph and poh scales. The easiest way to perform the calculation on a scientific calculator is to enter the hydrogen ion concentrations, press the log key ( not the ln key, which is natural logarithm), and then take the negative of the value. Here is a table of ph values of common chemicals. Web with this ph calculator, you can determine the ph of a solution in a few ways. Ph = 14 − poh = 14 − 10.5 = 3.5. Hydrogen & hydroxide ion concentration calculator. Web definitions of ph, poh, and the ph scale. Determine the hydronium ion concentration and poh from ph. Exponential numbers should be entered using e to represent 10^. The ph value is an essential factor in chemistry, medicine, and daily life. Determine the ph of acidic and basic solutions. Web with this ph calculator, you can determine the ph of a solution in a few ways. By definition, a strong acid is any substance that is good at donating an h + ion to water. Determine the hydronium ion concentration and poh from ph. As we have seen, [h3o+] [ h 3 o +] and [oh−] [ o h. The ph scale is the range of value s from 0 to 14 that describes the acidity or basicity of a solution. Web the scale runs from 0 (most acidic) to 14 (most alkaline or basic), where 7 is neutral ph. This is because of rounding off. Perform calculations relating ph and poh. By the end of this section, you. The constant of water determines the range of the ph scale. Calculating the ph of a strong acid or base solution. Explain the characterization of aqueous solutions as acidic, basic, or neutral. Determine the ph of acidic and basic solutions. Perform calculations relating ph and poh. Web if ph > 7, then the solution is basic. Web the ph and poh values of some common substances at 25 °c are shown in this chart. Poh is related to ph and can be easily calculated from ph. This is known as the ph p h scale. Web define ph and poh. Here is a table of ph values of common chemicals. Ph = 14 − poh = 14 − 10.5 = 3.5. By definition, a strong acid is any substance that is good at donating an h + ion to water. Web definitions of ph, poh, and the ph scale. Explain the characterization of aqueous solutions as acidic, basic, or neutral. Determine the ph of acidic and basic solutions. This is because of rounding off. Explain the characterization of aqueous solutions as acidic, basic, or neutral. By definition, a strong acid is any substance that is good at donating an h + ion to water. The ph scale is the range of value s from 0 to 14 that describes the. Calculating the ph of a strong acid or base solution. (f) you might not get exactly 1.0 x 10¯ 14 if you multiply a [h 3 o +] and an [oh¯] from the same problem, say from the answers in exercise #3 or #4. You can use ph p h to make a quick determination whether a. Express hydronium and. Calculation of ph from [ h 3 o + ] what is the ph of stomach acid, a solution of \(\ce{hcl}\) with a hydronium ion. Web it indicates how acidic or basic a solution is. The relationship between acid strength and the ph of a solution. Express hydronium and hydroxide ion concentrations on the ph and poh scales. Web definitions. Explain the characterization of aqueous solutions as acidic, basic, or neutral. (f) you might not get exactly 1.0 x 10¯ 14 if you multiply a [h 3 o +] and an [oh¯] from the same problem, say from the answers in exercise #3 or #4. Web define ph and poh. Explain the characterization of aqueous solutions as acidic, basic, or. Hydrogen & hydroxide ion concentration calculator. This is known as the ph p h scale. Determine the ph of acidic and basic solutions. To understand what the pk w is, it is important to understand first what the p means in poh, and ph. The relationship between acid strength and the ph of a solution. Calculating the ph of a strong acid or base solution. The ph value is an essential factor in chemistry, medicine, and daily life. Determine the ph of acidic and basic solutions. Explain the characterization of aqueous solutions as acidic, basic, or neutral. Web define ph and poh. It is a quantitative measure of the acidity or alkalinity of a solution. The first column is labeled “left bracket h subscript 3 o superscript plus right bracket (m).” Explain the characterization of aqueous solutions as acidic, basic, or neutral. Ph = 14 − poh = 14 − 10.5 = 3.5. Web define ph and poh. For a solution with a poh of 10.5, the corresponding ph is approximately 3.5.
8.3 pH and pOH Chemistry for Chemical Engineers

Ph And Poh Scale
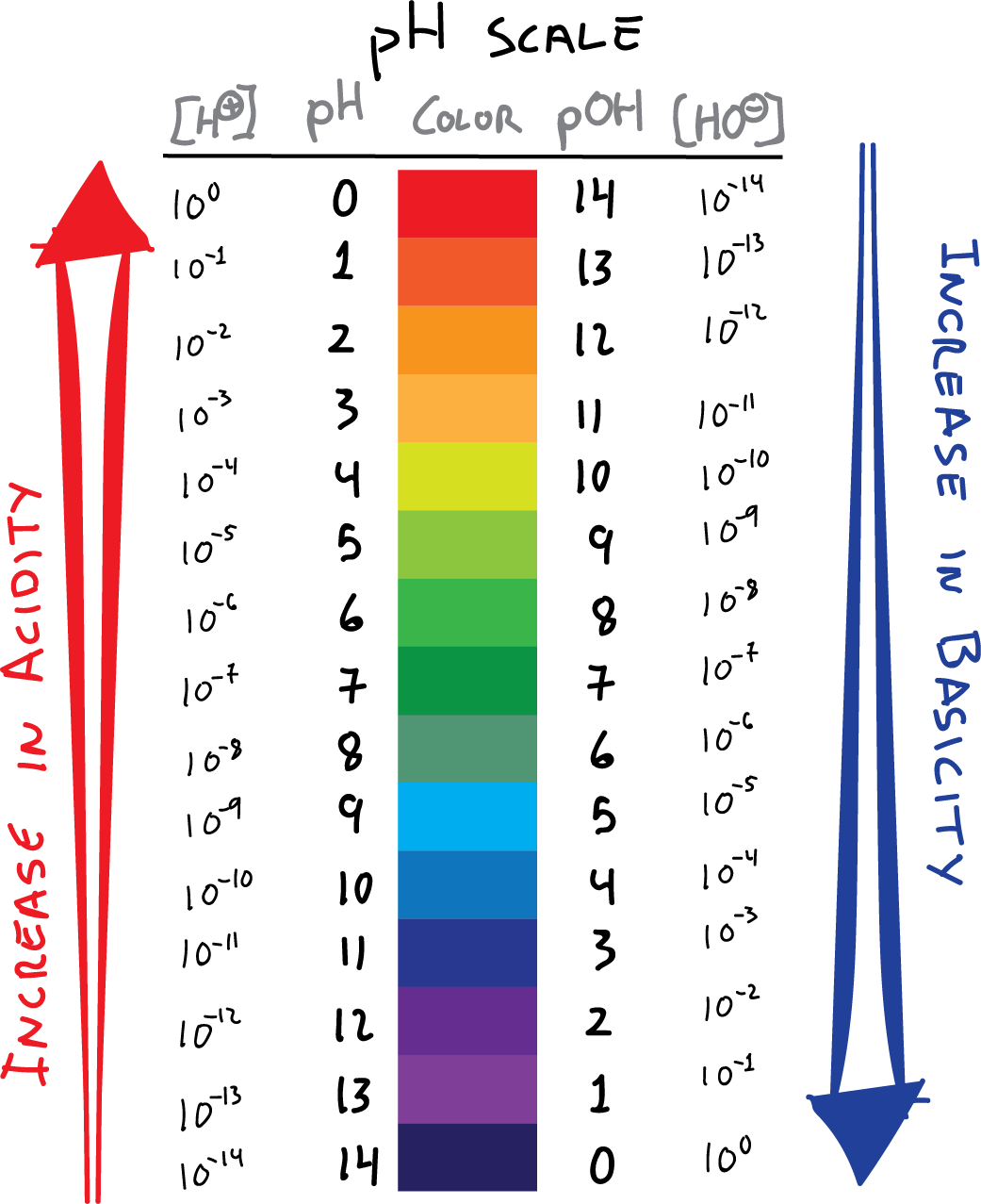
What is the pH Scale?, Examples, Video Chemistry Online
Ph Poh Chart For Calculations
[Solved] . 8. Complete the chart PH POH Acid or Base H* Concentration

pH and pOH chart Diagram Quizlet
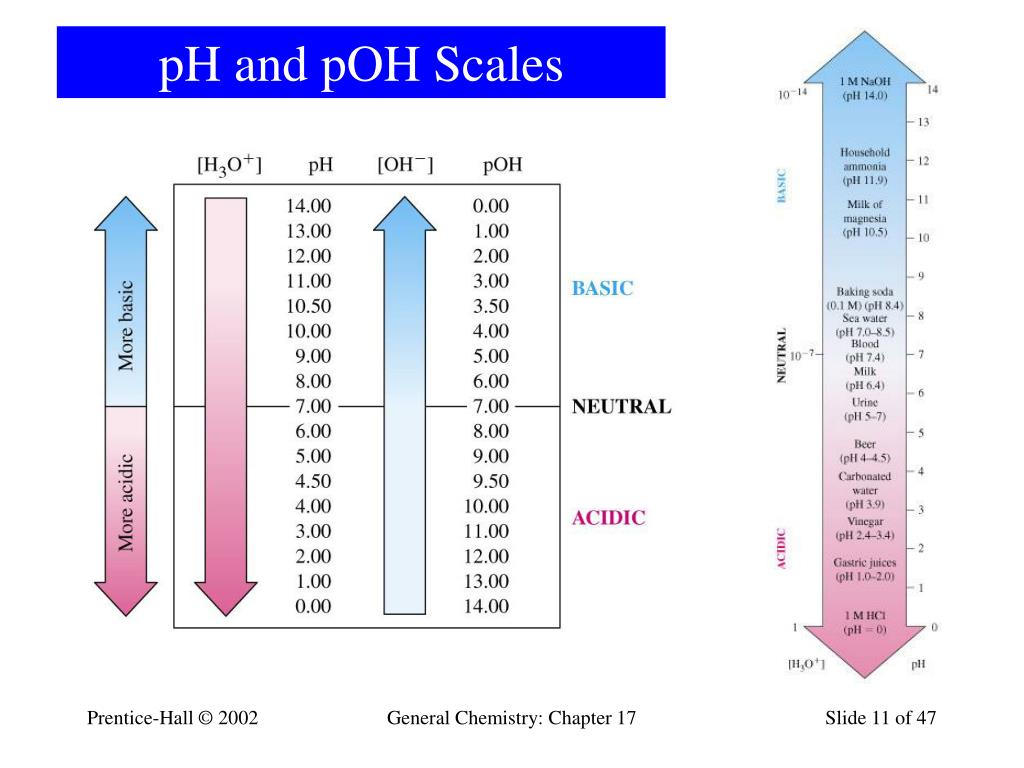
PPT Chapter 17 Acids and Bases PowerPoint Presentation, free
PH pOH Chart PDF
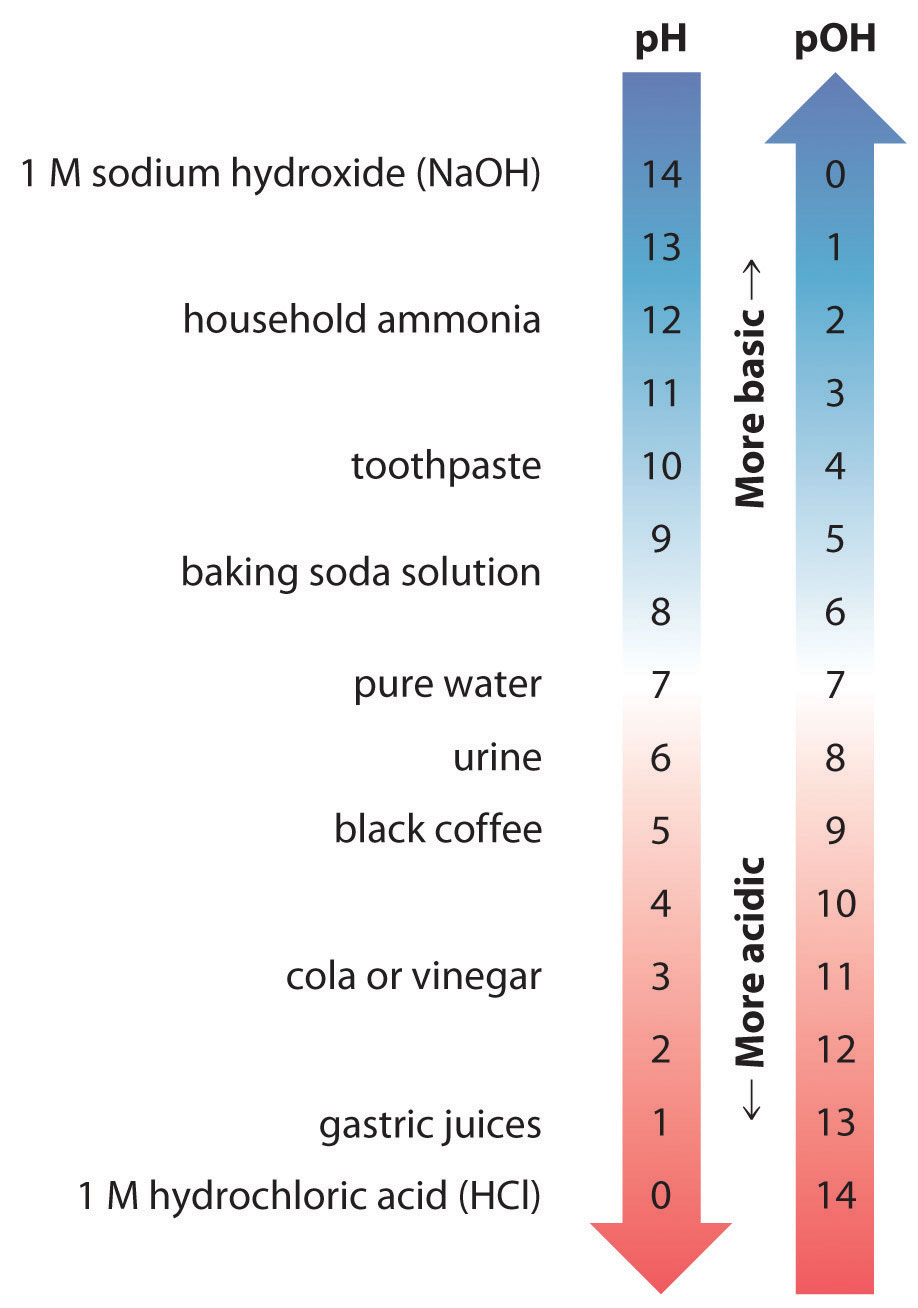
2.3 SelfIonization of Water and the pH Scale Chemistry LibreTexts
Ph Definition Chemistry
These Examples Demonstrate The Straightforward Conversion Between Ph And Poh Using The Relationship Ph + Poh = 14 In Aqueous Solutions At 25°C.
Web When A Ph, A Poh, Or K W Is Shown, No Unit Of M Will Appear.
To Calculate Ph, First Convert Concentration To Molarity.
Express Hydronium And Hydroxide Ion Concentrations On The Ph And Poh Scales.
Related Post:
