Ph Indicator Color Chart
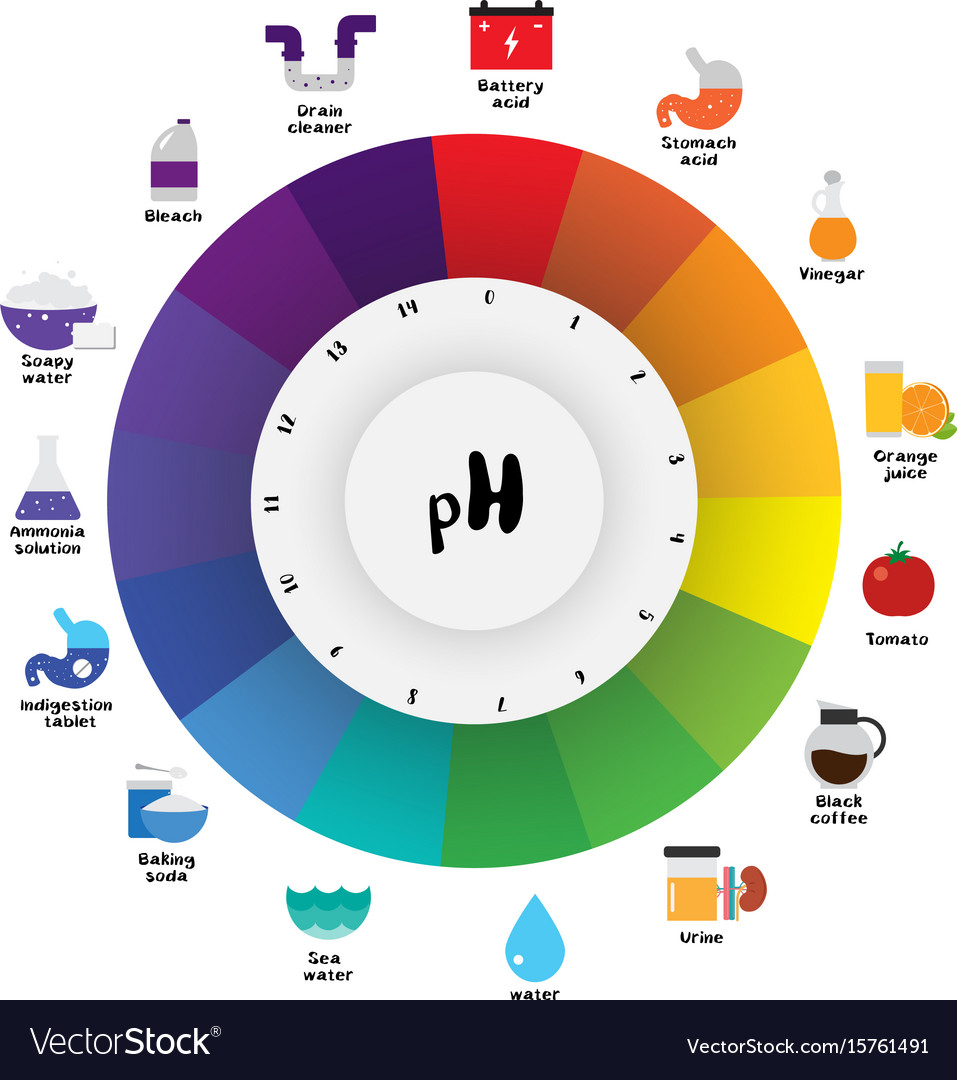
Ph Indicator Color Chart - Web students will see a demonstration of a color change using universal ph indicator. They are usually weak acids or bases, but their conjugate base or acid forms have different colors due to differences in their absorption spectra. Web the ph indicator chart is colorful and beautiful, helping with the selection of the best indicator for any ph range and also the identification of color changes. Web ph indicators are weak acids that exist as natural dyes and indicate the concentration of h + ( h3o +) ions in a solution via color change. When universal indicator is added to a solution, the color change can indicate the approximate ph of the solution. Learn how to make universal indicator solution and interpret ph results. A ph value is determined from the negative logarithm of this concentration and is used to indicate the acidic, basic, or neutral character of the substance you are testing. Web a ph indicator is a halochromic chemical compound added in small amounts to a solution so the ph (acidity or basicity) of the solution can be determined visually or spectroscopically by changes in absorption and/or emission properties. Print out one or all of the accompanying sheets and allow learners to explore the colours of the ph scale by using the associated numbers to match the colours of the universal indicator. Phenolphthalein ranges from colourless to pink. Web common indicators, such as phenolphthalein, methyl red, and bromothymol blue, are used to indicate ph ranges of approximately 8 to 10, 4.5 to 6, and 6 to 7.5, respectively. Web ph indicators are weak acids that exist as natural dyes and indicate the concentration of h + ( h3o +) ions in a solution via color change. Web a. Bromothymol blue ranges from yellow to blue. Students will change the concentrations of an acid and a base and use universal indicator to test the ph of the resulting solutions. Learn how to make universal indicator solution and interpret ph results. Colour matching charts are supplied with the specific test strips purchased. The ph indicator chart displays the colors of. Web figure \(\pageindex{2}\) shows the approximate ph range over which some common indicators change color and their change in color. Web an indicator is usually some weak organic acid or base dye that changes colors at definite ph values. Luckily, once you understand how the color coding works, reading a ph strip is easy! Is a substance that changes colour. Web the audience of users, the decision whether to determine a titration error for a particular color change and the experimental environment must be considered when deciding what quantity is best to use. Web students will see a demonstration of a color change using universal ph indicator. To familiarise learners with the range of colours produced by universal indicator. Luckily,. Web ph indicators are weak acids that exist as natural dyes and indicate the concentration of h + ( h3o +) ions in a solution via color change. Methyl red ranges from red to yellow. In addition, some indicators (such as thymol blue) are polyprotic acids or bases, which change color twice at. To familiarise learners with the range of. Web students will see a demonstration of a color change using universal ph indicator. Web if you’ve never used a ph strip, though, it might look like just a plain strip of paper, and the colorful chart looks like something you’d see in art class. Web 0 2 4 6 ph 8 10 12 14 ph indicator colours universal indicator. Play this game to see where different common substances appear on the ph. Web the colors from yellow to red indicate an acidic solution, colours blue to violet indicate an alkaline solution and a green colour indicates that a solution is neutral. In addition, some indicators (such as thymol blue) are polyprotic acids or bases, which change color twice at.. Web figure \(\pageindex{2}\) shows the approximate ph range over which some common indicators change color and their change in color. Acids cause universal indicator solution to change from green toward red. Web the colors from yellow to red indicate an acidic solution, colours blue to violet indicate an alkaline solution and a green colour indicates that a solution is neutral.. Students will change the concentrations of an acid and a base and use universal indicator to test the ph of the resulting solutions. Web indicators are substances whose solutions change color due to changes in ph. Web the audience of users, the decision whether to determine a titration error for a particular color change and the experimental environment must be. Make sure your strips test the range you need. Bromothymol blue ranges from yellow to blue. You can prepare homemade indicators from. Web ph can be also be measured using an indicator and comparing the colour with a comparison chart. Web ph indicators are weak acids that exist as natural dyes and indicate the concentration of h + ( h3o. Play this game to see where different common substances appear on the ph. Luckily, once you understand how the color coding works, reading a ph strip is easy! In addition, some indicators (such as thymol blue) are polyprotic acids or bases, which change color twice at. In conclusion, selecting the right ph indicator involves considering factors such as accuracy, ph. Bases cause universal indicator to change from green toward purple. To introduce learners to the concept of acids and alkalis. Is a substance that changes colour when it is added to acidic or alkaline solutions. Ph indicators generally change color over a range of two ph units. They are usually weak acids or bases, but their conjugate base or acid forms have different colors due to differences in their absorption spectra. Phenolphthalein ranges from colourless to pink. Web common indicators, such as phenolphthalein, methyl red, and bromothymol blue, are used to indicate ph ranges of approximately 8 to 10, 4.5 to 6, and 6 to 7.5, respectively. Web a ph indicator is a halochromic chemical compound added in small amounts to a solution so the ph (acidity or basicity) of the solution can be determined visually or spectroscopically by changes in absorption and/or emission properties. Whether a solution is acidic or basic can be measured on the ph scale. You can prepare homemade indicators from. Web an indicator is usually some weak organic acid or base dye that changes colors at definite ph values. When universal indicator is added to a solution, the color change can indicate the approximate ph of the solution.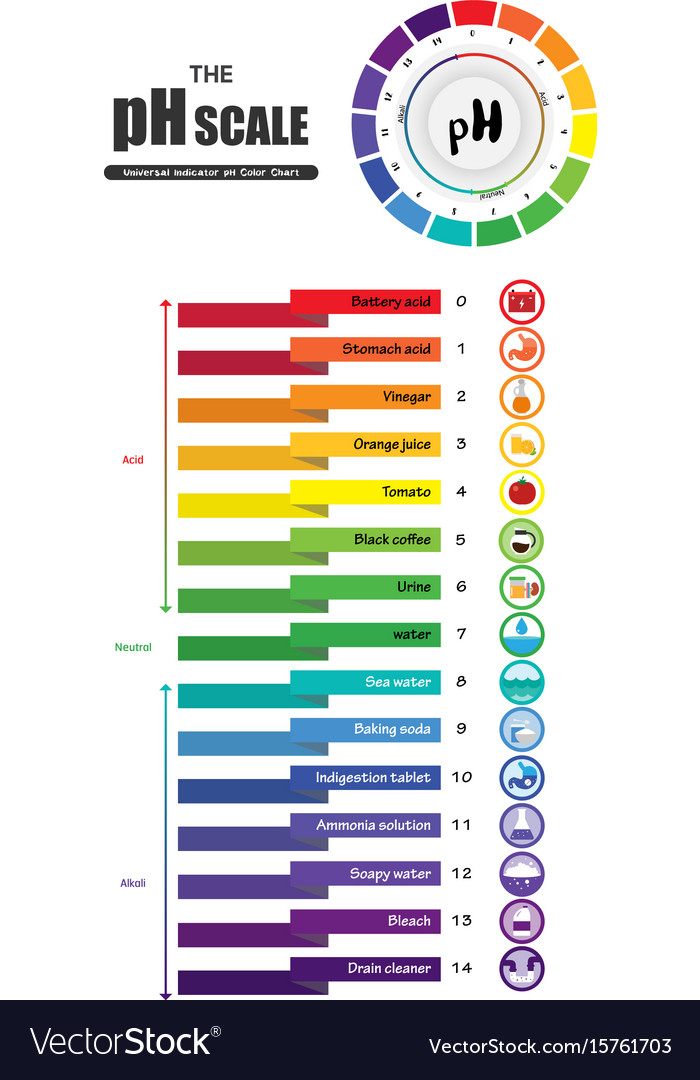
Ph scale universal indicator color chart Vector Image

Universal Indicator Ph Color Chart Scientific Stock Vector (Royalty
Ph Indicator Chart Colors And Ranges Images and Photos finder
Ph scale universal indicator color chart Vector Image
![]()
The PH Scale Universal Indicator PH Color Chart Diagram Stock Vector

Ph scale universal indicator color chart Vector Image

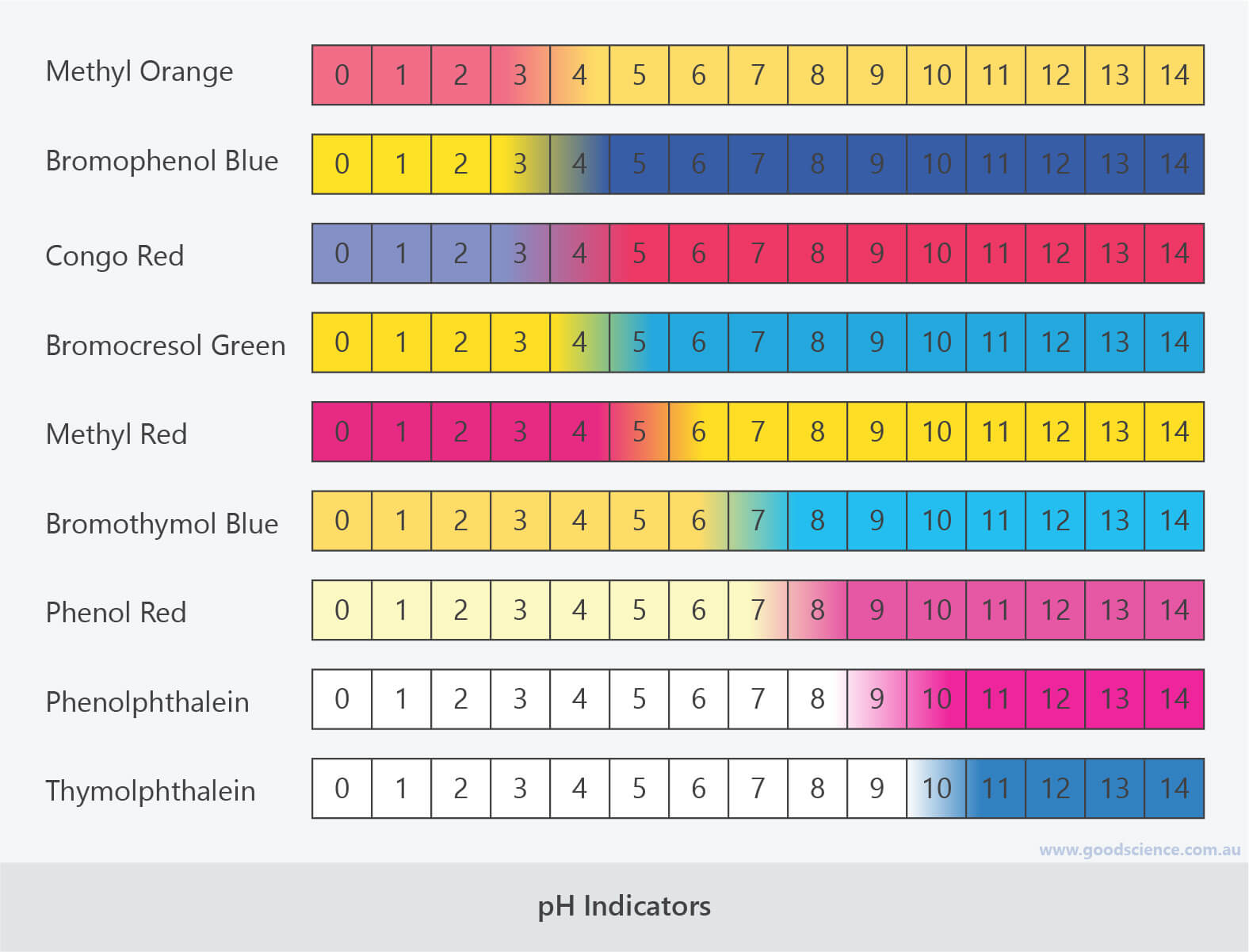
pH Indicator Chart Colors and Ranges
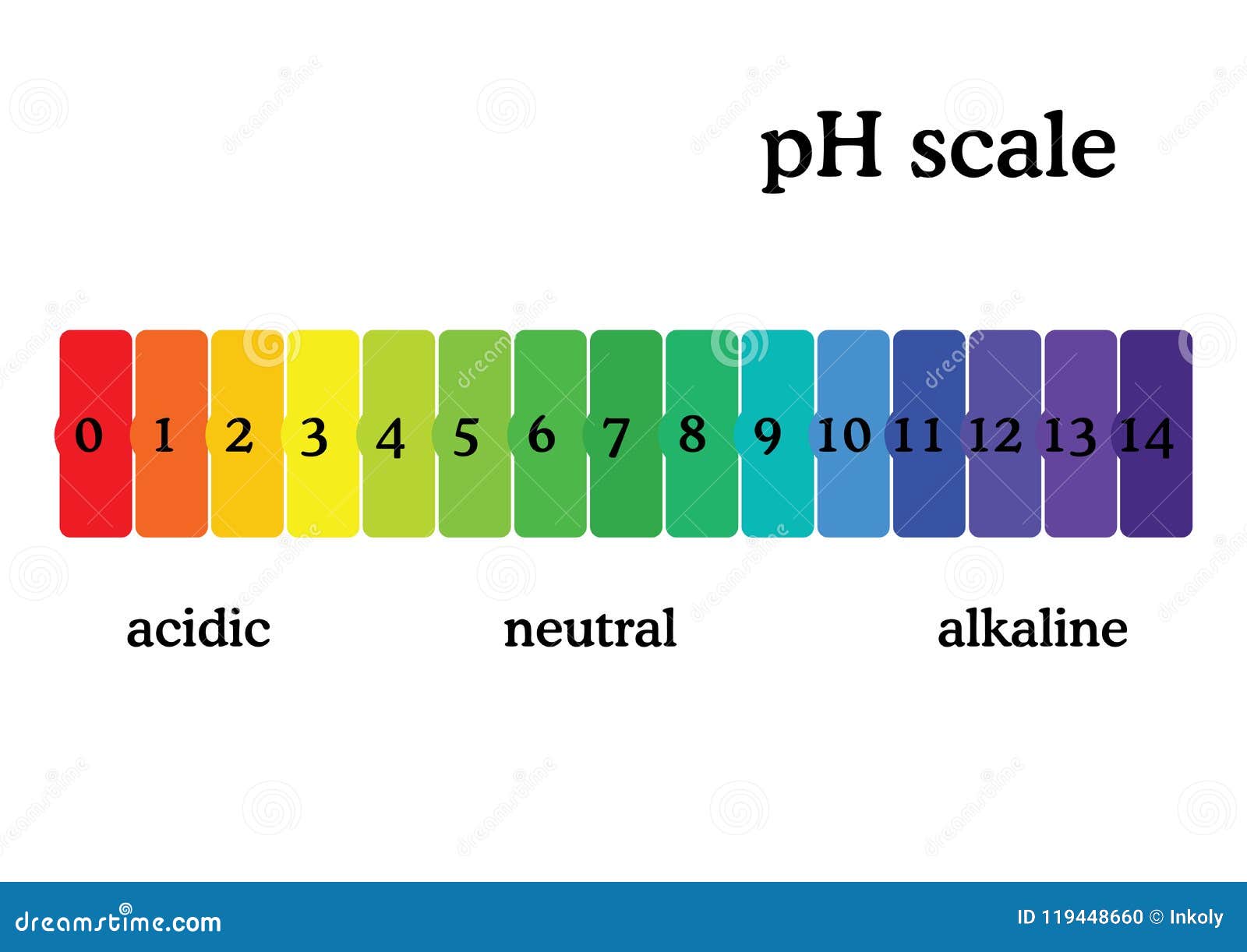
PH Scale Diagram with Corresponding Acidic or Alcaline Values

Ph scale universal indicator color chart Vector Image

Ph scale universal indicator color chart Vector Image
Web Figure \(\Pageindex{2}\) Shows The Approximate Ph Range Over Which Some Common Indicators Change Color And Their Change In Color.
Bromothymol Blue Ranges From Yellow To Blue.
A Ph Value Is Determined From The Negative Logarithm Of This Concentration And Is Used To Indicate The Acidic, Basic, Or Neutral Character Of The Substance You Are Testing.
Students Will See An Animation Showing That Water Molecules Interact And Separate Into The H 3 O + Ion And The Oh − Ion.
Related Post: