Nacl Drawing
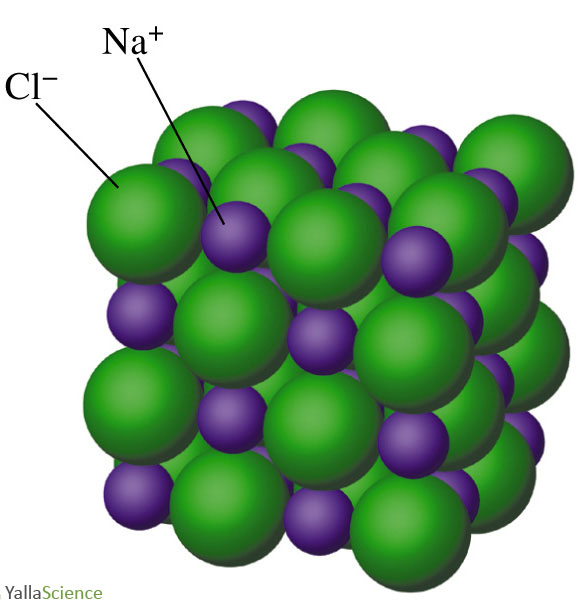
Nacl Drawing - When drawing a lewis dot structure, we are always attempting to achieve an electron count at which all of the atoms involved are stable and (usually) have full octets. Is to draw the unit cell in slices along one of the unit cell axes. To be consistent with the law of conservation of mass, the before reaction and after. Since this is a diatomic molecule with only two atoms, selecting the central atom with the lowest electronegativity is not. Sodium chloride, also known as salt, common salt, table salt or halite, is an ionic compound with the chemical formula nacl, representing a 1:1 ratio of sodium and chloride ions. Web to draw the bohr diagram for nacl, we should first draw the individual diagrams for both na and cl. It has 1:1 stoichiometry ratio of na:cl with a molar mass of 58.4 g/mol. Rock salt (nacl) figure \(\pageindex{5}\): The cell looks the same whether you start with anions or cations on the corners. Web rock salt ( nacl nacl) is an ionic compound that occurs naturally as white crystals. We are also attempting to establish a structure with the smallest formal charge possible. The cell looks the same whether you start with anions or cations on the corners. It also explains why cesium chloride has a different structure from sodium. Web steps to draw lewis structure of sodium chloride. A given chemical reaction can be represented using a particulate. With that, the formula of sodium chloride is nacl. In this model, it is a representation of the structure of sodium chloride, an ionic network. Since they’re from opposite sides of the periodic table, they form an ionic compound. Since sodium is in group i of the periodic table, it forms ions with charge of +1. Web to draw the. Web nacl lewis structure mainly involved 2 element i.e. Sodium chloride, also known as salt, common salt, table salt or halite, is an ionic compound with the chemical formula nacl, representing a 1:1 ratio of sodium and chloride ions. The first and second valence shells are completely full, since their 2 and 8 electrons only take up the first 10. Sodium (na) metal and chlorine (cl) atom. We are also attempting to establish a structure with the smallest formal charge possible. The cell looks the same whether you start with anions or cations on the corners. It's made of up of a sodium positively charged ion or cation, and a chloride negatively charged ion. Count all the valence electrons. In its lewis structure na is bonded to cl with ionic bond. Since sodium is in group i of the periodic table, it forms ions with charge of +1. Thus it is a diatomic molecule. In this model, it is a representation of the structure of sodium chloride, an ionic network. Web the nacl structure is a good example of. The first and second valence shells are completely full, since their 2 and 8 electrons only take up the first 10 of sodium's 11 electrons. In its lewis structure na is bonded to cl with ionic bond. It has 1:1 stoichiometry ratio of na:cl with a molar mass of 58.4 g/mol. Is to draw the unit cell in slices along. The cell looks the same whether you start with anions or cations on the corners. Thus it is a diatomic molecule. Since they’re from opposite sides of the periodic table, they form an ionic compound. Since this is a diatomic molecule with only two atoms, selecting the central atom with the lowest electronegativity is not. Count all the valence electrons. Compounds with the sodium chloride. Since they’re from opposite sides of the periodic table, they form an ionic compound. The intramolecular bonding is ionic, as it involves the transfer of electrons from sodium to chlorine, and bonds ions through electrostatic forces. When drawing a lewis dot structure, we are always attempting to achieve an electron count at which all of. Web the nacl structure is a good example of the latter. All octahedral holes in a cubic close packing are occupied by counterions. The intramolecular bonding is ionic, as it involves the transfer of electrons from sodium to chlorine, and bonds ions through electrostatic forces. Since this is a diatomic molecule with only two atoms, selecting the central atom with. Web learn how to draw the structure of sodium chloride in a simple and easy way with this video tutorial. A sodium atom has 11 electrons. The general rule is to first isolate all of the. Chlorine is in group 17, so it has seven valence. A given chemical reaction can be represented using a particulate diagram, in which the. Sodium = 1 valence electron. The atomic number of na is 11, so it has 11 electrons. Web how to draw lewis structure of nacl. The structure of nacl is formed by repeating the face centered cubic unit cell. We are also attempting to establish a structure with the smallest formal charge possible. Web check me out: Sodium chloride is the primary salt in seawater and in the extracellular fluid of many multicellular organisms. It also explains why cesium chloride has a different structure from sodium. With that, the formula of sodium chloride is nacl. A sodium atom has 11 electrons. In this model, it is a representation of the structure of sodium chloride, an ionic network. All octahedral holes in a cubic close packing are occupied by counterions. The intramolecular bonding is ionic, as it involves the transfer of electrons from sodium to chlorine, and bonds ions through electrostatic forces. Thus it is a diatomic molecule. Since this is a diatomic molecule with only two atoms, selecting the central atom with the lowest electronegativity is not. To be consistent with the law of conservation of mass, the before reaction and after.
Structure of NaCl (Sodium chloride) YouTube

Structure of Sodium Chloride (NaCl) Formation of NaCl How to draw
Chemistry model salt molecule diatomic sodium chlorine NaCl scientific

How To Draw Sodium Chloride (NaCl) Diagram? YouTube

Free vector graphic Crystal Structure, Nacl, Chemical Free Image on

Nacl structure,nacl crystal structure,how to draw nacl structure

Sodium Chloride Nacl Molecular Cube HighRes Vector Graphic Getty Images

Structure of NaCl Easy trick to draw sodium chloride structure (Full
Structure Of NaCl (Sodium Chloride) chegos.pl

Class1112th। How to draw Nacl crystal structure? Nacl क्रिस्टल संरचना
It's Made Of Up Of A Sodium Positively Charged Ion Or Cation, And A Chloride Negatively Charged Ion.
Sodium (Na) Metal And Chlorine (Cl) Atom.
The First And Second Valence Shells Are Completely Full, Since Their 2 And 8 Electrons Only Take Up The First 10 Of Sodium's 11 Electrons.
Count All The Valence Electrons.
Related Post: