Melting Point Chart
Melting Point Chart - Melting points for some metals and alloys: The melting point is the temperature at which a substance changes state from solid to liquid at atmospheric pressure. The tabular chart on the right is arranged by melting point. The melting point of a substance depends on pressure and is usually specified at standard pressure. Melting point of copper alloys. The element with the lowest melting point is helium, with a melting point of 0.95 k (−272.20 °c, −457.96 °f) at 2.5 mpa pressure. Web live scores for every 2024 ncaaf season game on espn. Web refer to this chart for the melting points of various metals and alloys. We say that such a body melts. Web the melting point of an element is basically the energy required to change the state of an element from its solid state to its liquid state. Web the melting point of an element is basically the energy required to change the state of an element from its solid state to its liquid state. The melting point is the temperature at which the vapour pressure of the solid and the liquid are the same and the presssure totals one atmosphere. Melting points of common materials. At its. Densities, specific heats, viscosities and more. We say that such a body melts. The element with the lowest melting point is helium, with a melting point of 0.95 k (−272.20 °c, −457.96 °f) at 2.5 mpa pressure. Web melting point is the temperature at which a substance changes from solid to liquid state. The typical behavior of an impure solid. Web for chemistry students and teachers: The melting point of a substance depends on pressure and is usually specified at standard pressure. The melting point is also referred to as liquefaction point, solidus, or liquidus. Web this melting point chart contains the most common metals used in manufacturing in order of their melting points, and are more commonly used due. Web melting point in the periodic table of elements. It is also a temperature at which a solid (crystal) turns into a liquid. Web a substance's melting point depends on pressure and is usually specified at standard pressure in reference materials. Web refer to this chart for the melting points of various metals and alloys. When considered as the temperature. Web refer to this chart for the melting points of various metals and alloys. When considered as the temperature of the reverse change (from liquid to solid), it is referred to as the freezing point. At its melting point, the disruptive vibrations of the particles of the solid overcome the attractive forces operating within the solid. Web the melting point. The typical behavior of an impure solid containing two components is summarized by the general phase diagram in figure 6.7a. Web melting point in the periodic table of elements. Web the melting point is the temperature at which a solid changes into a liquid. Web refer to this chart for the melting points of various metals and alloys. Web for. Densities, specific heats, viscosities and more. Web this melting point chart contains the most common metals used in manufacturing in order of their melting points, and are more commonly used due to factors like strength and corrosion resistance. Web melting point is the temperature at which a substance changes from solid to liquid state. Web sodium chloride melts at 801°c.. Melting point of common materials. In the following table, the use row is the value recommended for use in other wikipedia pages in order to maintain consistency across content. We say that such a body melts. Web a substance's melting point depends on pressure and is usually specified at standard pressure in reference materials. Web melting point is the temperature. We say that such a body melts. Web the melting point of an element is basically the energy required to change the state of an element from its solid state to its liquid state. The chemical element with the lowest melting point is helium and the element with the highest melting point is carbon. Web the melting point is the. Melting point, temperature at which the solid and liquid forms of a pure substance can exist in equilibrium. When a substance melts, some of the attractive forces between particles are broken or loosened. As with boiling points, the melting point of a solid is dependent on the strength of those attractive forces. The typical behavior of an impure solid containing. At the melting point the solid and liquid phase exist in equilibrium. Melting points for some metals and alloys: Melting point of copper alloys. There is a lot going on in this graph, so it is often easier to divide it into three sections. When considered as the temperature of the reverse change (from liquid to solid), it is referred to as the freezing point. Image showing periodicity of the chemical elements for melting point in. For example, copper melts at 1084°c and steel has a melting point that ranges from 1371°c to 1593°c. When a substance melts, some of the attractive forces between particles are broken or loosened. Melting points of common materials. On the other hand, ice (solid h 2 o) is a molecular compound whose molecules are held together by hydrogen bonds, which is effectively a strong example of an interaction between two permanent dipoles. The unity used for the melting point is celsius (c). Web melting point in the periodic table of elements. The melting point of a substance depends on pressure and is usually specified at standard pressure. The chemical element with the lowest melting point is helium and the element with the highest melting point is carbon. Web a substance's melting point depends on pressure and is usually specified at standard pressure in reference materials. Properties of gases, fluids and solids.
Chart of Melting Points of Elements Stock Photo Alamy

Melting points table of the elements
Metal Melting Points Chart

Melting Points Of Metals Chart

Melting Point Definition and List

Melting point chart Ericvisser
Metal Melting Point Chart Metals Silver

Minerals Melting Point Chart
All Metal Melting Points Metals Nickel
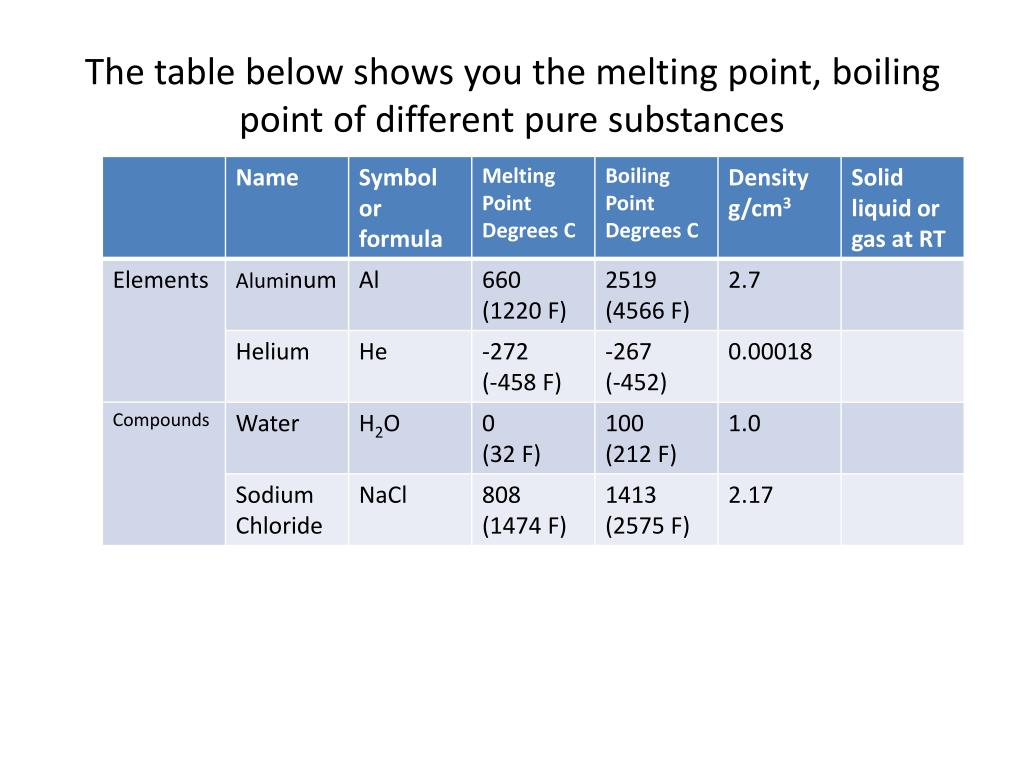
Melting Points And Boiling Points
As Heat Is Applied To A Solid, Its Temperature Will Increase Until The Melting Point Is Reached.
The Typical Behavior Of An Impure Solid Containing Two Components Is Summarized By The General Phase Diagram In Figure 6.7A.
The Graph Shows How Melting Points And Boiling Points Vary Across Period 3.
Melting Point Of Aluminum Alloys.
Related Post:

