Ionization Energy Chart Periodic Table
Ionization Energy Chart Periodic Table - The first molar ionization energy applies to the neutral atoms. Ionization energy is also a periodic trend within the periodic table. How would you write a chemical equation representing the third ionization energy for lithium? Web ionization energy chart of all the elements is given below. Web for each atom, the column marked 1 is the first ionization energy to ionize the neutral atom, the column marked 2 is the second ionization energy to remove a second electron from the +1 ion, the column marked 3 is the third ionization energy to remove a third electron from the +2 ion, and so on. Ionization energy generally increases moving from left to right across an element period (row). Web what is ionization energy. There are trends that match the structure of the periodic table. Web ionization energies increase from left to right across each row, with discrepancies occurring at ns 2 np 1 (group 13), ns 2 np 4 (group 16), and ns 2 (n − 1)d 10 (group 12) electron configurations. Web what is ionization energy? Learn its chemical equation, values, trends across a period & down a group, & exception. Web explore how ionization energy changes with atomic number in the periodic table of elements via interactive plots. The energy required to remove an electron is the ionization energy. Web what is ionization energy. This version of the periodic table shows the first ionization energy. Atomic # transition metals lanthanides. The ionization energy differs for each atom. Web the periodic table of the elements (with ionization energies) 1. Web explore how ionization energy changes with atomic number in the periodic table of elements via interactive plots. Web the first ionization energy, second ionization energy as well as third ionization energy of the elements are given. Web on the periodic table, first ionization energy generally increases as you move left to right across a period. Alkali metals alkaline earth metals. How would you write a chemical equation representing the third ionization energy for lithium? The first molar ionization energy applies to the neutral atoms. Web explore how ionization energy changes with atomic number in the periodic. How would you write a chemical equation representing the third ionization energy for lithium? Alkali metals alkaline earth metals. Web ionization energy chart of all the elements is given below. Web locate the elements in the periodic table. Web the first ionization energy, second ionization energy as well as third ionization energy of the elements are given in this chart. Learn its chemical equation, values, trends across a period & down a group, & exception. Web for each atom, the column marked 1 is the first ionization energy to ionize the neutral atom, the column marked 2 is the second ionization energy to remove a second electron from the +1 ion, the column marked 3 is the third ionization energy. There are trends that match the structure of the periodic table. Web explore how ionization energy changes with atomic number in the periodic table of elements via interactive plots. Learn its chemical equation, values, trends across a period & down a group, & exception. Atomic # transition metals lanthanides. Across a period, ionization energy tends to increase. There are trends that match the structure of the periodic table. Chemical elements listed by ionization energy. The reason is that the atomic radius tends to decrease moving across a period. Also, learn first & second ionization energies. It assumes that you know about simple atomic orbitals, and can write electronic structures for simple atoms. The elements of the periodic table sorted by ionization energy. The ionization energy differs for each atom. How would you write a chemical equation representing the third ionization energy for lithium? What does the ionization energy quantitatively measure about an atom? Web explore how ionization energy changes with atomic number in the periodic table of elements via interactive plots. Web ionization energy chart of all the elements is given below. This is due to increasing nuclear charge, which results in the outermost electron being more strongly bound to the nucleus. The elements of the periodic table sorted by ionization energy. Want to join the conversation? Alkali metals alkaline earth metals. Alkali metals alkaline earth metals. The elements of the periodic table sorted by ionization energy. Web the periodic table of the elements (with ionization energies) 1. Web ionization energy displays a trend on the periodic table. This is due to increasing nuclear charge, which results in the outermost electron being more strongly bound to the nucleus. Also, learn first & second ionization energies. First ionization energies generally decrease down a column. Web what is ionization energy. Web these tables list values of molar ionization energies, measured in kj⋅mol −1. Web what is ionization energy? What does the ionization energy quantitatively measure about an atom? Web interactive periodic table showing names, electrons, and oxidation states. Click on any element's name for further information on chemical properties, environmental data or health effects. This version of the periodic table shows the first ionization energy of (ie: This is due to increasing nuclear charge, which results in the outermost electron being more strongly bound to the nucleus. Ionization energy is also a periodic trend within the periodic table. Definition of ion and ionization energy, and trends in ionization energy across a period and down a group. Visualize trends, 3d orbitals, isotopes, and mix compounds. Which group of elements has the highest ionization energies? 1), in kj/mol, of selected elements. Web ionization energy chart of all the elements is given below.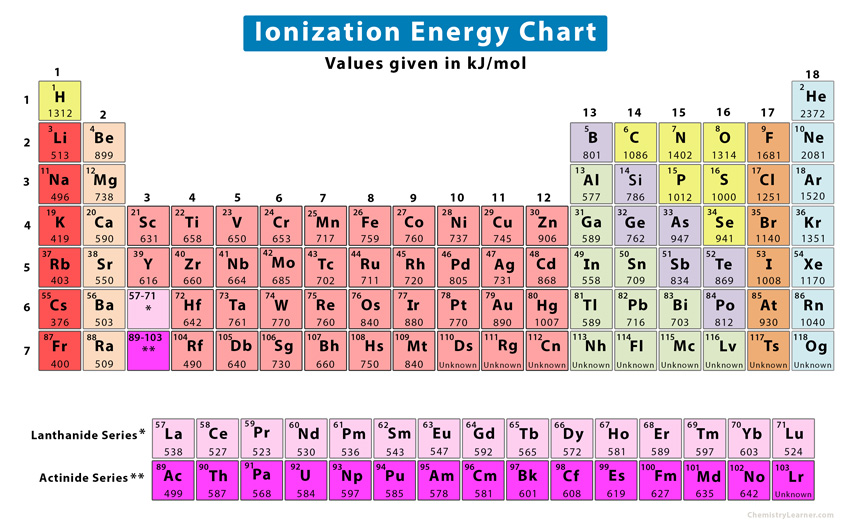
Ionization Energy Periodic Table Matttroy
Periodic Trends in Ionization Energy Chemistry Socratic
Ionization energy periodic table lopezguy
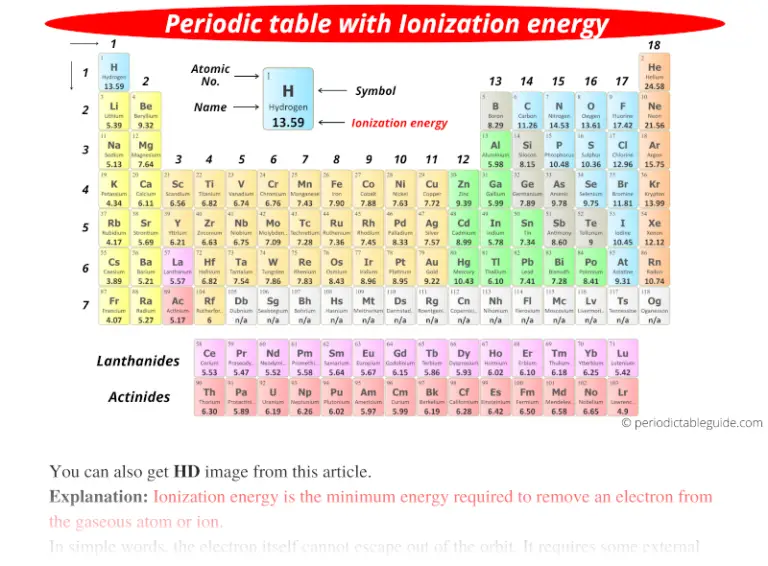
Periodic table with Ionization Energy Values (Labeled Image)
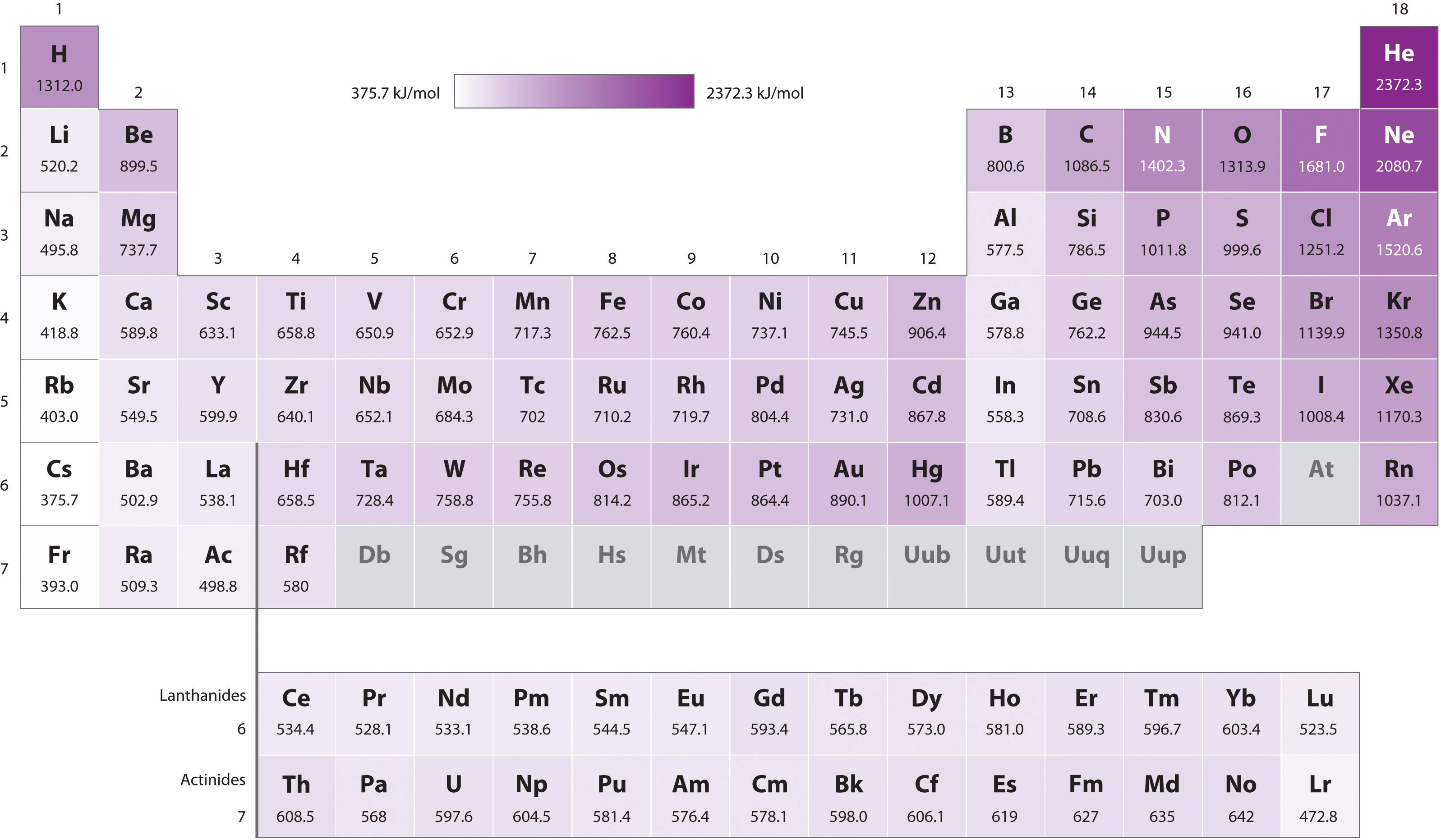
8.4 Ionization Energy Chemistry LibreTexts
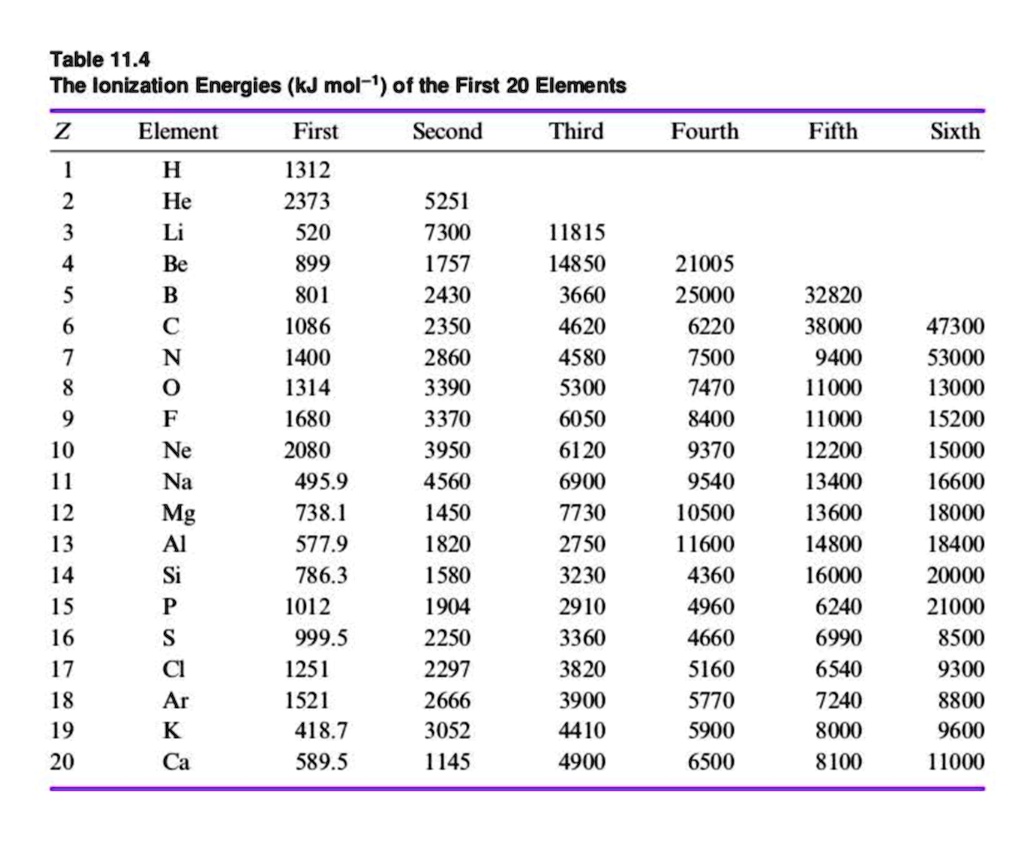
Periodic Table Of Elements First Ionization Energy Matttroy
Periodic Behavior Presentation Chemistry
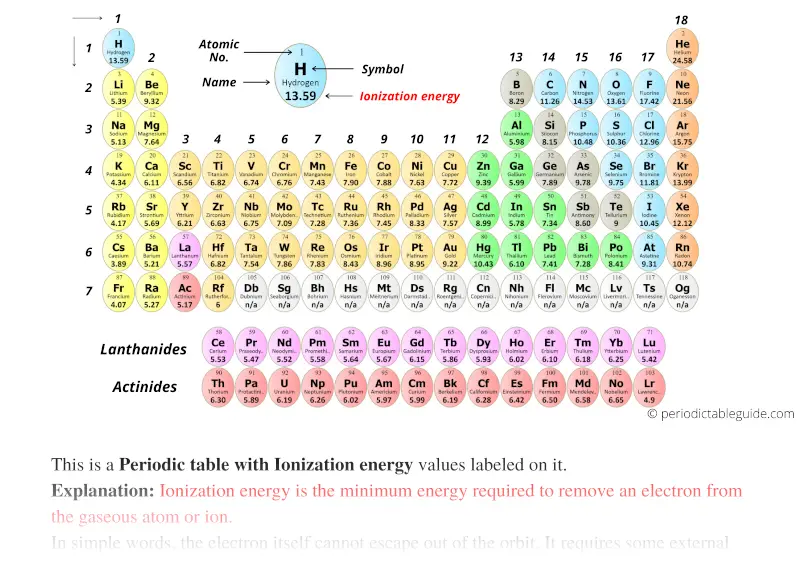
Periodic table with Ionization Energy Values (Labeled Image)
Periodic Trends in Ionization Energy CK12 Foundation

Ionization Energy Periodic Table
Web Explore How Ionization Energy Changes With Atomic Number In The Periodic Table Of Elements Via Interactive Plots.
This List Contains The 118 Elements Of Chemistry.
Web Ionization Is The Process Of Removing An Electron From A Neutral Atom (Or Compound).
It Assumes That You Know About Simple Atomic Orbitals, And Can Write Electronic Structures For Simple Atoms.
Related Post:
.PNG)
