Ionic Energy Chart
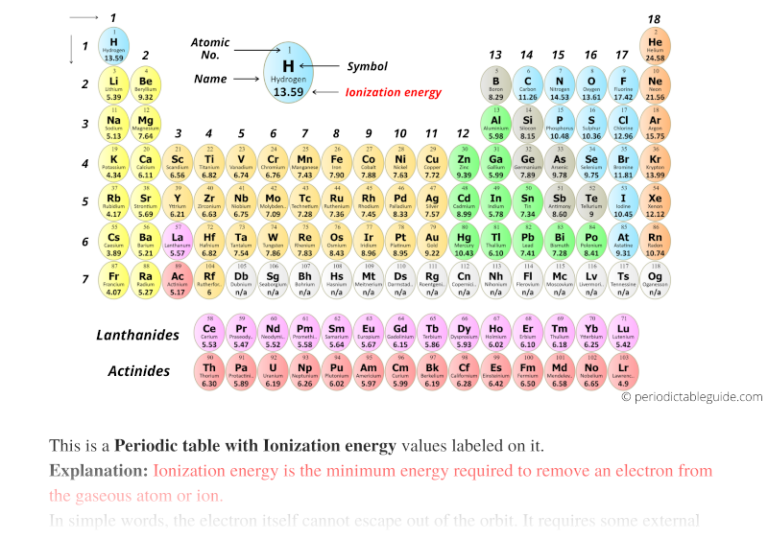
Ionic Energy Chart - This is the energy per mole necessary to remove electrons from gaseous atoms or atomic ions. Web nist atomic spectra database ionization energies form. Web for each atom, the column marked 1 is the first ionization energy to ionize the neutral atom, the column marked 2 is the second ionization energy to remove a second electron from the +1 ion, the column marked 3 is the third ionization energy to remove a third electron from the +2 ion, and so on. Want to join the conversation? Web ionization energy chart of all the elements is given below. \(i\) is therefore the energy required for the reaction First ionisation energy shows periodicity. X + is an ion of atom x with a single positive charge. Web these tables list values of molar ionization energies, measured in kj⋅mol −1. U can be calculated from the charges on the ions, the arrangement of the ions in the solid, and the internuclear distance. Web the values mentioned in the above periodic table is the first ionization energy and are given in electron volts (ev). The first molar ionization energy applies to the neutral atoms. Web definition of ion and ionization energy, and trends in ionization energy across a period and down a group. U can be calculated from the charges on the ions,. This is the energy per mole necessary to remove electrons from gaseous atoms or atomic ions. Web chemists define the ionization energy (\(i\)) of an element as the amount of energy needed to remove an electron from the gaseous atom \(e\) in its ground state. Want to join the conversation? Web table of polyatomic ions. Please report any accidental mistake. Web table of polyatomic ions. Web the 2nd ionization energy of the element m is a measure of the energy required to remove one electron from one mole of the gaseous ion m + image showing periodicity of the chemical elements for ionization energy: Web nist atomic spectra database ionization energies form. Web the lattice energy (u) of an ionic. When oppositely charged ions are brought together from r = ∞ to r = r0, the energy of the system is lowered (energy is released). Web this list contains the 118 elements of chemistry. U can be calculated from the charges on the ions, the arrangement of the ions in the solid, and the internuclear distance. Want to join the. Web in physics and chemistry, ionization energy (ie) is the minimum energy required to remove the most loosely bound electron of an isolated gaseous atom, positive ion, or molecule. Web ionization energy chart of all the elements is given below. This is the energy per mole necessary to remove electrons from gaseous atoms or atomic ions. Web the lattice energy. \(i\) is therefore the energy required for the reaction For a schematic overview of the periodic table of elements in chart form. That means that it varies in a repetitive way as you move through the periodic table. Web the first 20 elements. The first ionization energy, second ionization energy as well as third ionization energy of the elements are. Web the first 20 elements. Use average covalent bond energies to estimate enthalpies of reaction Web ionization energy chart of all the elements is given below. When oppositely charged ions are brought together from r = ∞ to r = r0, the energy of the system is lowered (energy is released). Want to join the conversation? For example, look at the pattern from li to ne, and then compare it with the identical pattern from na to ar. The 1st ionization energy of the element m is a measure of the energy required to remove one electron from one mole of the gaseous atoms m. Web in physics and chemistry, ionization energy (ie) is the minimum. This form provides access to nist critically evaluated data on ground states and ionization energies of atoms and atomic ions. Web ionization energy is a measure of the energy needed to pull a particular electron away from the attraction of the nucleus. What is meant by “first” ionization energy? The size of that attraction will be governed by: The 1st. The first ionization energy is quantitatively expressed as x(g) + energy x. For a schematic overview of the periodic table of elements in chart form. Want to join the conversation? This form provides access to nist critically evaluated data on ground states and ionization energies of atoms and atomic ions. The size of that attraction will be governed by: The first molar ionization energy applies to the neutral atoms. Web in physics and chemistry, ionization energy (ie) is the minimum energy required to remove the most loosely bound electron of an isolated gaseous atom, positive ion, or molecule. Web explore how ionization energy changes with atomic number in the periodic table of elements via interactive plots. For a schematic overview of the periodic table of elements in chart form. This form provides access to nist critically evaluated data on ground states and ionization energies of atoms and atomic ions. Image showing periodicity of the chemical elements for ionization energy: Web definition of ion and ionization energy, and trends in ionization energy across a period and down a group. U can be calculated from the charges on the ions, the arrangement of the ions in the solid, and the internuclear distance. The first ionization energy, second ionization energy as well as third ionization energy of the elements are given in this chart below. Want to join the conversation? X + is an ion of atom x with a single positive charge. Web chemists define the ionization energy (\(i\)) of an element as the amount of energy needed to remove an electron from the gaseous atom \(e\) in its ground state. When oppositely charged ions are brought together from r = ∞ to r = r0, the energy of the system is lowered (energy is released). In mehreren sprachenerfahrene journalistenmoderne plattformgenaue nachrichten On the periodic table, first ionization energy generally increases as you move left to right across a period. Web this list contains the 118 elements of chemistry.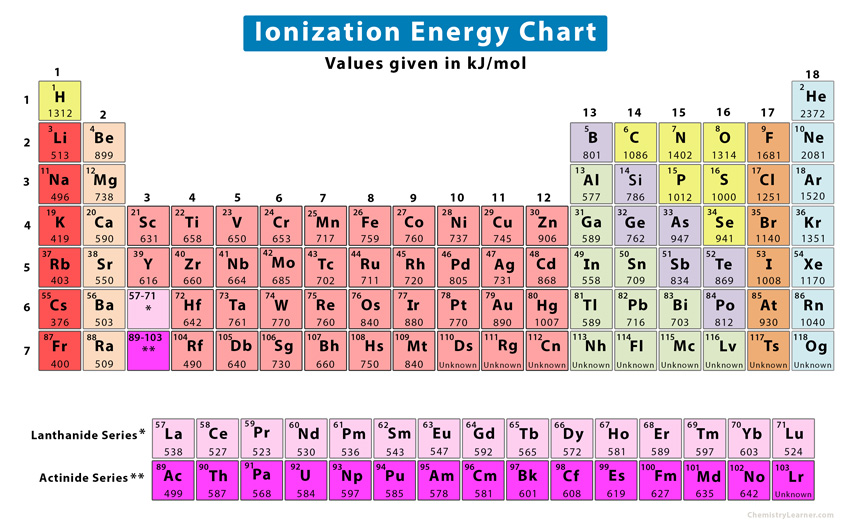
Among the Following Which Element Has the Lowest Ionization Energy
Periodic table with Ionization Energy Values (Labeled Image)
9.9 Periodic Trends Atomic Size, Ionization Energy, and Metallic
Calculate The Value Of The Third Ionization Energy Of Lithium How To
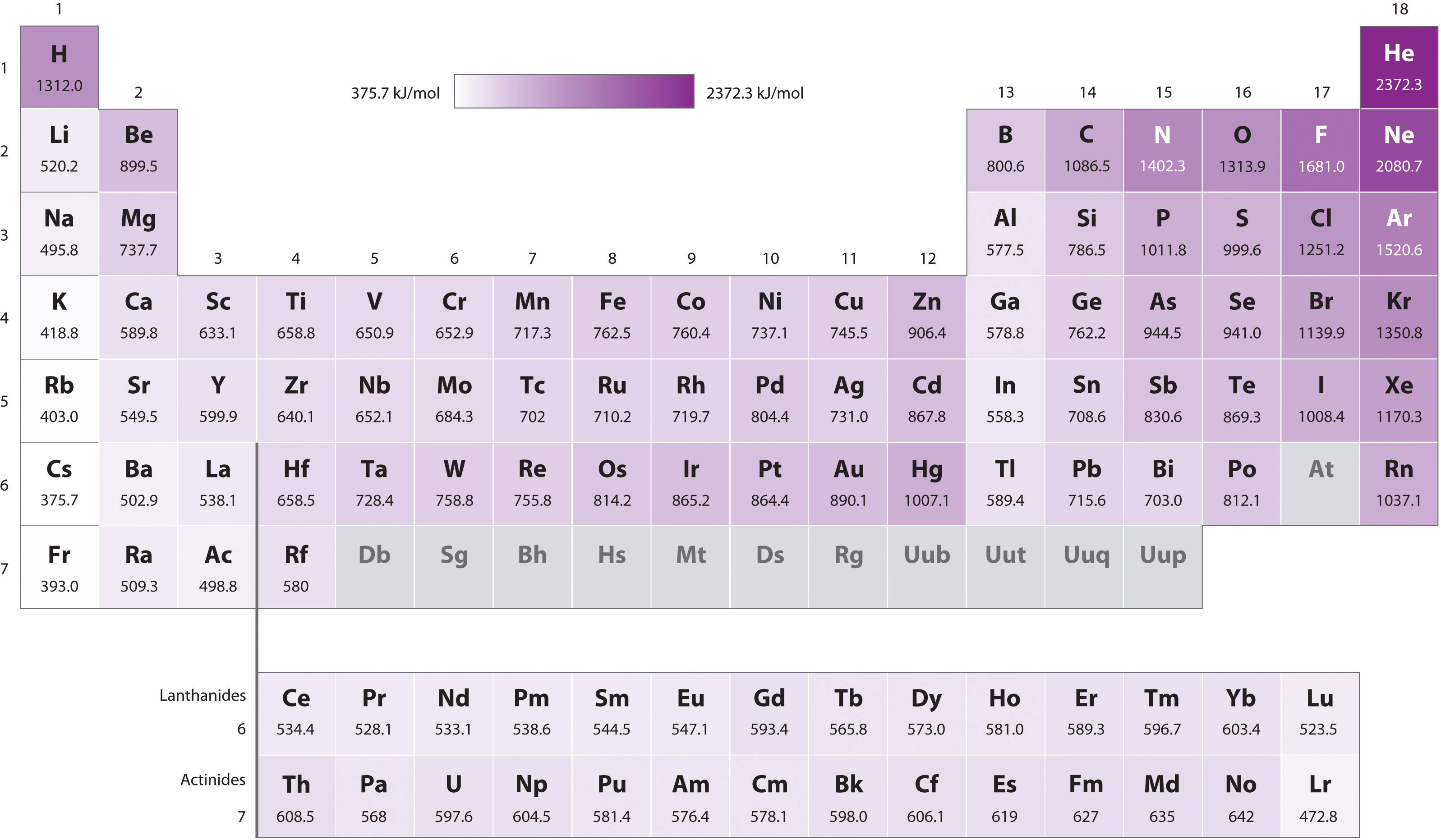
6.6 Ionization Energies Chemistry LibreTexts
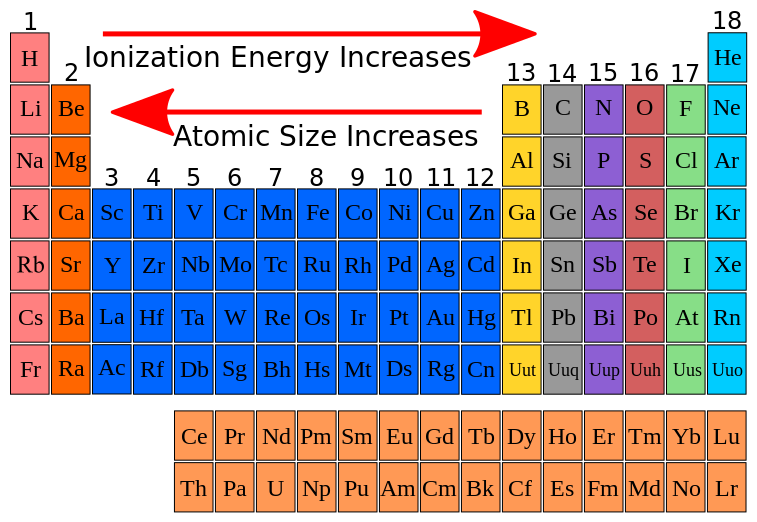
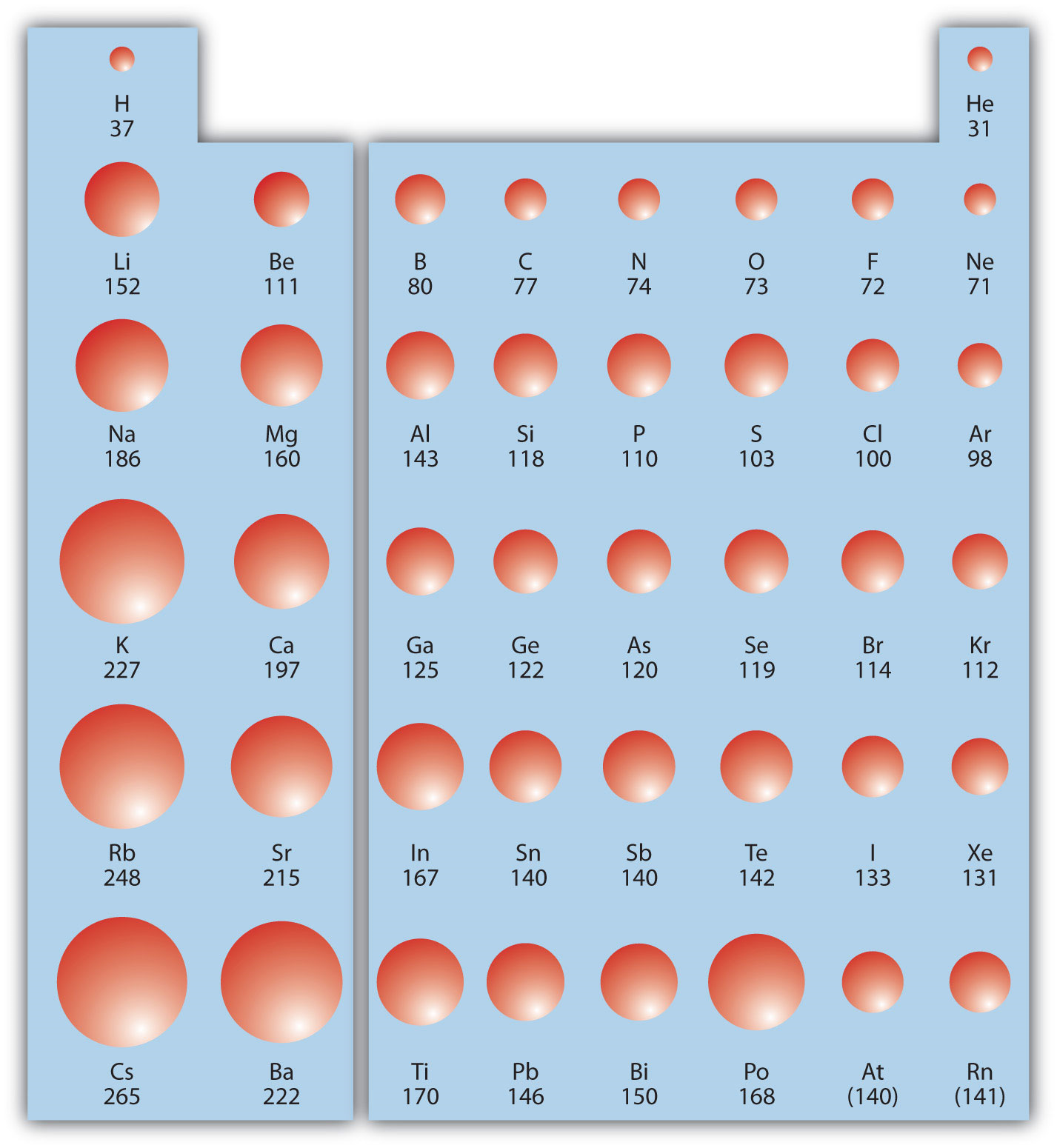
FileIonization energy atomic size.svg Wikimedia Commons
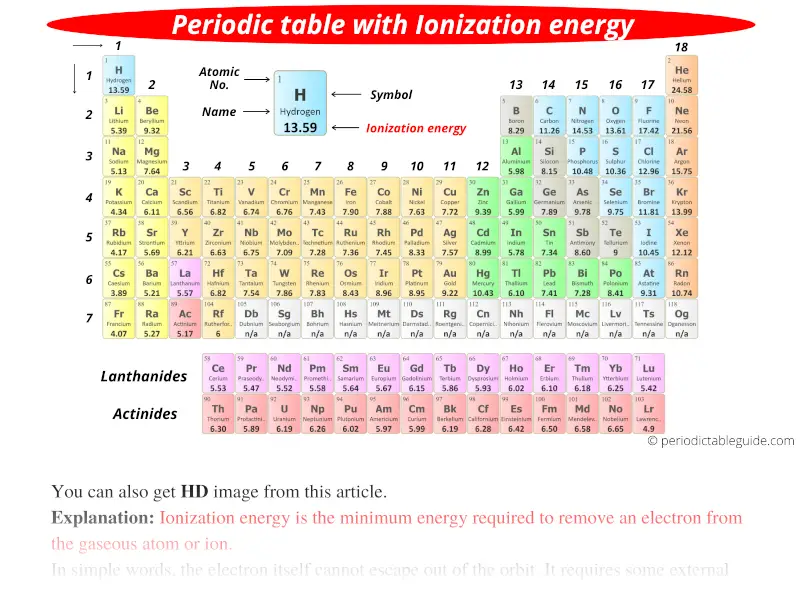
Periodic table with Ionization Energy Values (Labeled Image)
Periodic Behavior Presentation Chemistry
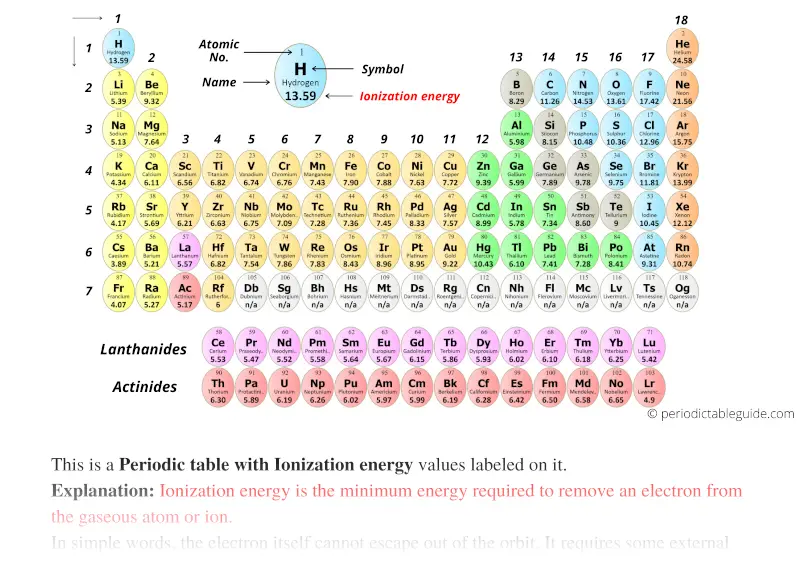
Periodic table with Ionization Energy Values (Labeled Image)
Periodic Trends in Ionization Energy CK12 Foundation
A High Value Of Ionization Energy Shows A High Attraction Between The Electron And The Nucleus.
2Nd In A Periodic Table Cityscape Style.
For Example, Look At The Pattern From Li To Ne, And Then Compare It With The Identical Pattern From Na To Ar.
Web We Summarize The Important Points About Ionic Bonding:
Related Post:
.PNG)
