How To Draw Hydrogen Bonds
How To Draw Hydrogen Bonds - Carbon is still bonded to these hydrogens but we're going to ignore them for our bond line structure. This video shows three examples of drawing for the formation of hydrogen bond. Web learn which types of molecules make hydrogen bonds and how to draw the hydrogen bonding interactions between two molecules of the same type and between two m. Web any molecule which has a hydrogen atom attached directly to an oxygen or a nitrogen is capable of hydrogen bonding. Web hydrogen bonds are strong intermolecular forces created when a hydrogen atom bonded to an electronegative atom approaches a nearby electronegative atom. (see the previous section for an explanation of hard and soft.) In a water molecule, an oxygen atom forms two bonds, one to each hydrogen atom. Web draw hydrogen bonds between one water molecule and four ethanol molecules using marvin sketch. For example, ethanoic acid would be shown in a. Describe the structure, such as it is, of liquid water. Web learn which types of molecules make hydrogen bonds and how to draw the hydrogen bonding interactions between two molecules of the same type and between two m. The key to understanding water’s chemical behavior is its molecular structure. Add a bond between two atoms:select a bond type (see below), point to an atom on the canvas, and then drag. Define and illustrate hydrogen bonds. Select a bond type (see below), point to the atom on the canvas, and then drag outwards from the atom.; Web let us use an aldehyde like acetaldehyde (ch3cho) and alcohol like ethanol (ch3ch2oh) as an example to draw a hydrogen bond. So, we leave those out in bond line structures. Web learn which types. Describe the roles of hydrogen bonding in proteins and in dna. It is also implemented as the command. Water is made up of two hydrogens and one oxygen atom, arranged in a tetrahedral shape. 10k views 3 years ago #exploding #teacher #chemical. Add a bond between two atoms:select a bond type (see below), point to an atom on the canvas,. Carbon is still bonded to these hydrogens but we're going to ignore them for our bond line structure. Web let us use an aldehyde like acetaldehyde (ch3cho) and alcohol like ethanol (ch3ch2oh) as an example to draw a hydrogen bond. It's a general rule that hard things like to bond with other hard things, and soft things like to bond. It's a general rule that hard things like to bond with other hard things, and soft things like to bond with other soft things. Add a bond between two atoms:select a bond type (see below), point to an atom on the canvas, and then drag the pointer to another atom.note that choosing a bond type and dragging the pointer on. This is because the oxygen atom, in addition to forming bonds with the hydrogen atoms, also carries two pairs of unshared electrons. So, we leave those out in bond line structures. First molecules has hydrogen attached to a highly electronegative atom (n,o,f). Describe the roles of hydrogen bonding in proteins and in dna. It is also implemented as the command. 10k views 3 years ago #exploding #teacher #chemical. This video shows three examples of drawing for the formation of hydrogen bond. A water molecule consists of two hydrogen atoms bonded to an oxygen atom, and its overall structure is bent. Web if you were to draw every carbon hydrogen bond in organic chemistry class it would take you forever. Sketch. (see the previous section for an explanation of hard and soft.) Two fluorine atoms can form a molecule of f 2 in the same fashion. 1) the first step is to draw them. 2) the next step is identifying hydrogen bond donors and the. Web polarity of water molecules. Web using lewis structures, we can represent this as follows: It is also implemented as the command. Web add a bond type to a single atom: The key to understanding water’s chemical behavior is its molecular structure. Here's what you must look for in order to be able to draw hydrogen bonds. You can simplify the formula by writing, for example, ch 3 or ch 2 instead of showing all these bonds. This video shows three examples of drawing for the formation of hydrogen bond. Web hydrogen bonds are strong intermolecular forces created when a hydrogen atom bonded to an electronegative atom approaches a nearby electronegative atom. Describe the roles of hydrogen. First molecules has hydrogen attached to a highly electronegative atom (n,o,f). Web hydrogen bonds are strong intermolecular forces created when a hydrogen atom bonded to an electronegative atom approaches a nearby electronegative atom. 1) the first step is to draw them. Add a bond between two atoms:select a bond type (see below), point to an atom on the canvas, and then drag the pointer to another atom.note that choosing a bond type and dragging the pointer on the. The key to understanding water’s chemical behavior is its molecular structure. (hydrogen bond donor) second molecule has a lone pair of electrons on a small highly electronegative atom (n,o,f). This is because the oxygen atom, in addition to forming bonds with the hydrogen atoms, also carries two pairs of unshared electrons. Carbon is still bonded to these hydrogens but we're going to ignore them for our bond line structure. Web let us use an aldehyde like acetaldehyde (ch3cho) and alcohol like ethanol (ch3ch2oh) as an example to draw a hydrogen bond. It's a general rule that hard things like to bond with other hard things, and soft things like to bond with other soft things. A hydrogen b hydrogen chloride c. So, we leave those out in bond line structures. Web explain what is meant by hydrogen bonding and the molecular structural features that bring it about. 10k views 3 years ago #exploding #teacher #chemical. A water molecule consists of two hydrogen atoms bonded to an oxygen atom, and its overall structure is bent. 2) the next step is identifying hydrogen bond donors and the.
Hydrogen Bonds In Water Explained Intermolecular Forces YouTube
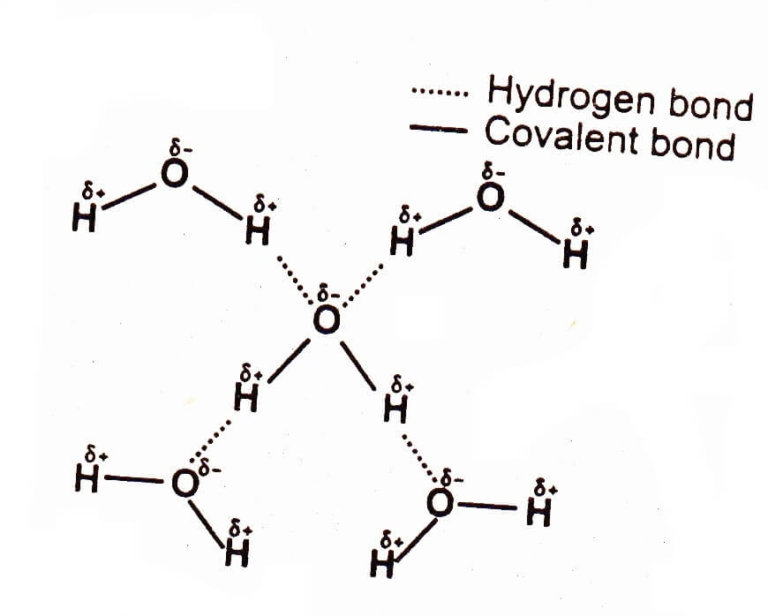
Hydrogen Bonding Chemistry Skills
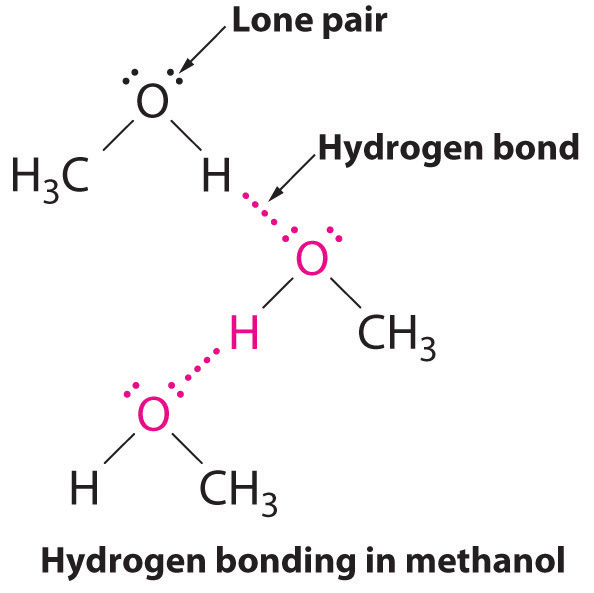
Intermolecular Forces
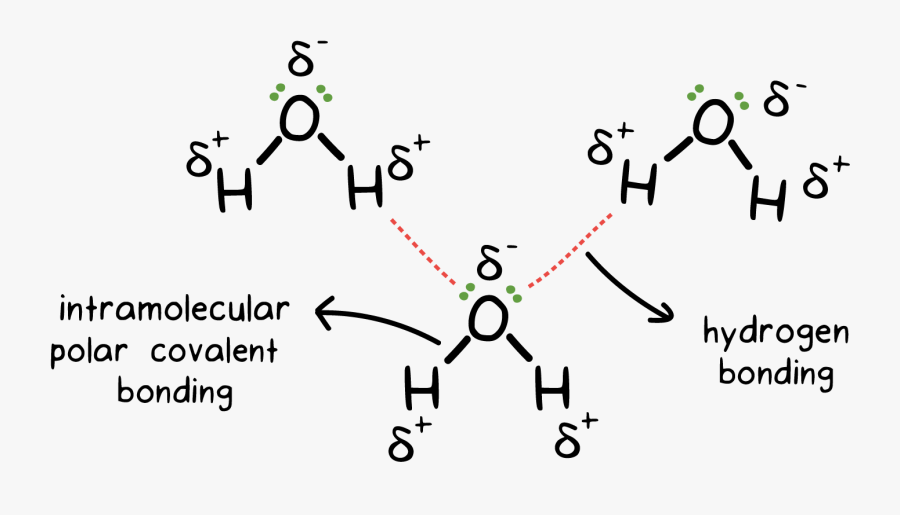
H2o Drawing Chemical Bond Intermolecular Hydrogen Bonding In Water
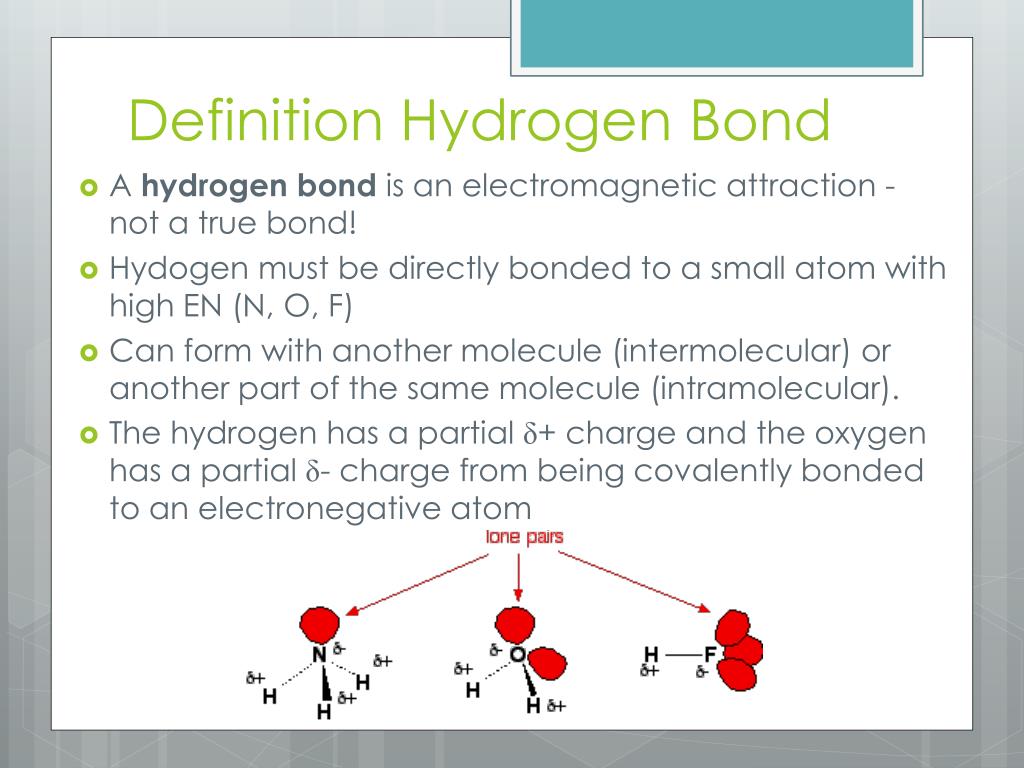
PPT Hydrogen Bonding PowerPoint Presentation, free download ID3887591

LabXchange

How To Draw Hydrogen Bonds Askworksheet

Hydrogen bonding

Hydrogen Bonding American Chemical Society

Hydrogen Bonding Diagram
Describe The Structure, Such As It Is, Of Liquid Water.
Web Science > Ap®︎/College Biology > Chemistry Of Life > Structure Of Water And Hydrogen Bonding.
Web Add A Bond Type To A Single Atom:
Describe The Roles Of Hydrogen Bonding In Proteins And In Dna.
Related Post: