How To Draw Hybrid Orbitals
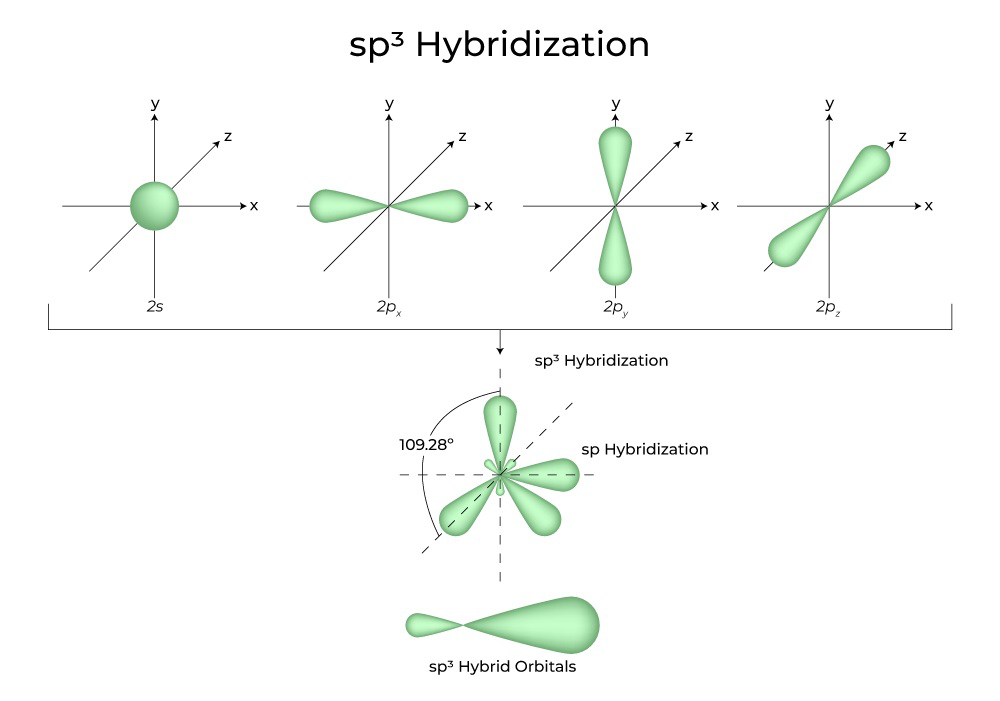
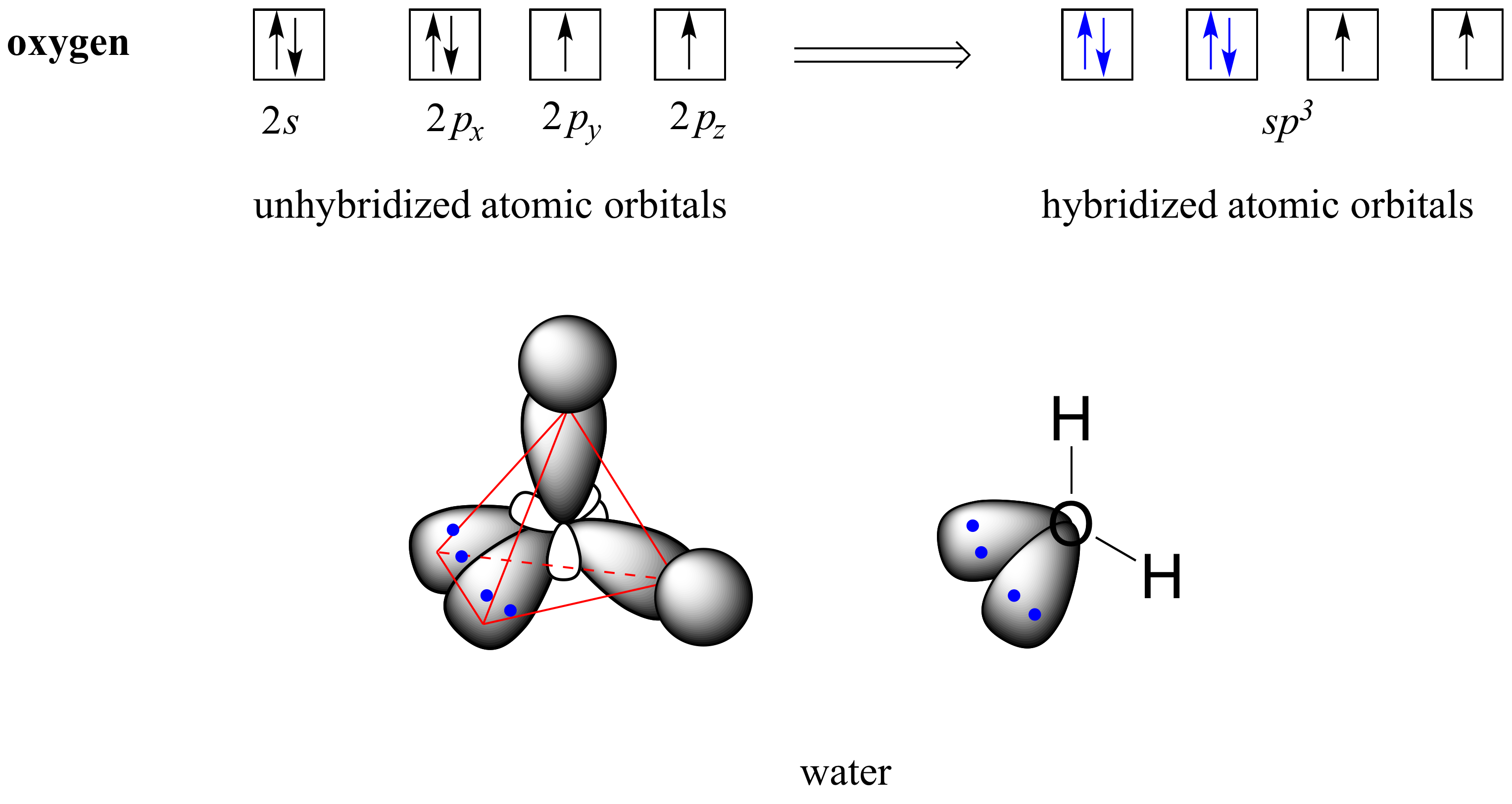
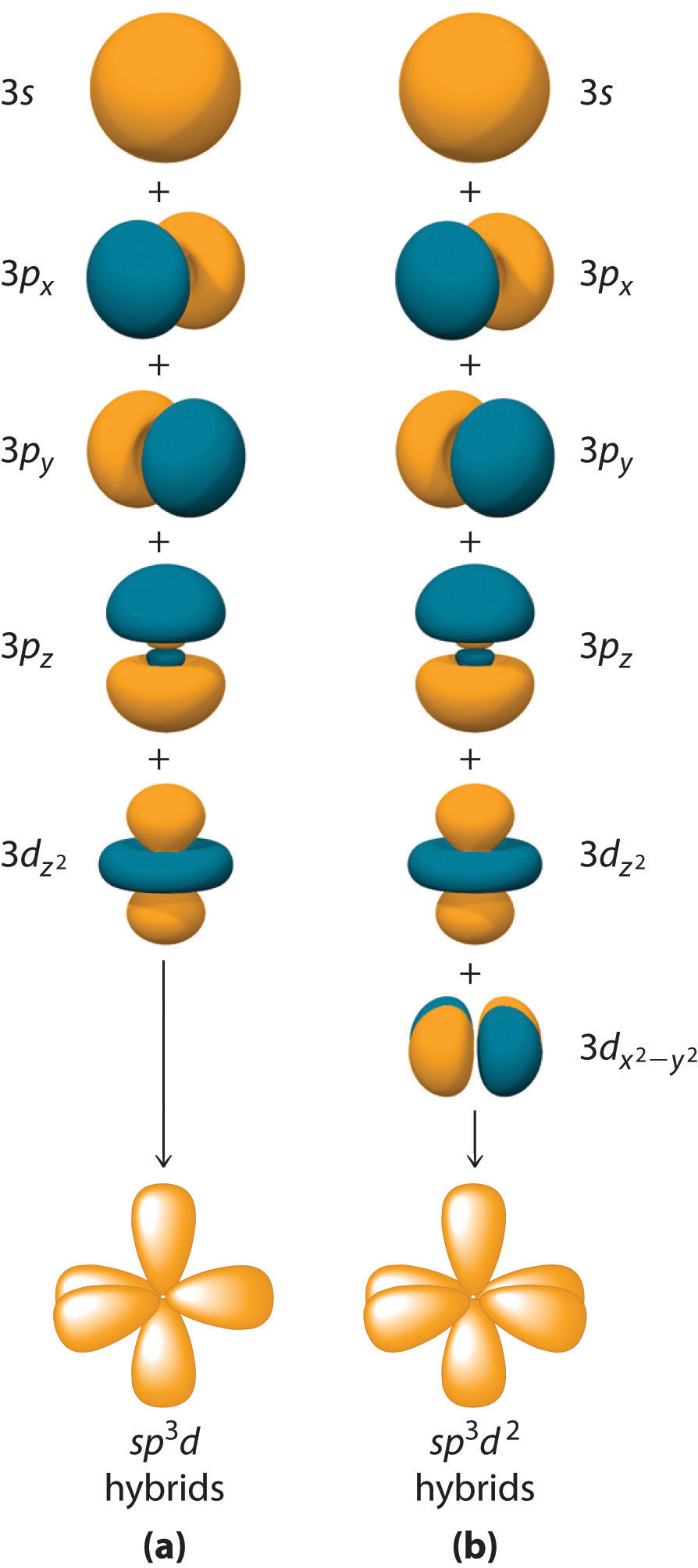
How To Draw Hybrid Orbitals - Draw the valence electron configuration for the ground state: Web we can find the hybridization of an atom in a molecule by either looking at the types of bonds surrounding the atom or by calculating its steric number. Let me draw them for. Web actually, you can view it as there are three 2p orbitals and each of them can hold two electrons, so it can hold a total of six electrons in the 2p orbitals. Web in sp hybridization, one s orbital and one p orbital hybridize to form two sp orbitals, each consisting of 50% s character and 50% p character. So first i'll draw the. This is the steric number (sn) of the central atom. Promote one s orbital electron to a half. Describe the hybrid orbitals used in the formation of bonding for each atom in some carbon containing compounds. It explains how to find the hybridization of. In sp² hybridization, one s orbital and two p orbitals hybridize to form three sp² orbitals, each consisting of 33% s character and 67% p character. Web steps to draw hybridized orbitals: The carbon atoms of c2h2 are sp hybridized. Web actually, you can view it as there are three 2p orbitals and each of them can hold two electrons,. Web steps to draw hybrid orbital diagrams for a molecule. 134k views 3 years ago. This makes it a challenge to draw,. It explains how to find the hybridization of. Draw the valence electron configuration for the ground state: Let me draw them for. In this video, we use. Sp 3 hybrid orbitals are oriented at bond angle of 109.5 o. Web steps to draw hybridized orbitals: In sp² hybridization, one s orbital and two p orbitals hybridize to form three sp² orbitals, each consisting of 33% s character and 67% p character. Describe the hybrid orbitals used in the formation of bonding for each atom in some carbon containing compounds. This makes it a challenge to draw,. Let me draw them for. Web we can find the hybridization of an atom in a molecule by either looking at the types of bonds surrounding the atom or by calculating its steric number. This. Web we can find the hybridization of an atom in a molecule by either looking at the types of bonds surrounding the atom or by calculating its steric number. Web steps to draw hybridized orbitals: This type of hybridization is required. Read through the provided information, and sketch the lewis dot diagram of the provided compound. Web steps to draw. Sp 3 hybrid orbitals are oriented at bond angle of 109.5 o. Web count the number of lone pairs + the number of atoms that are directly attached to the central atom. Web so let me draw what it would look like, or our best visual, or our best ability to kind of conceptualize what the orbitals around the carbon. Read through the provided information, and sketch the lewis dot diagram of the provided compound. This is the steric number (sn) of the central atom. Web steps to draw hybridized orbitals: Web actually, you can view it as there are three 2p orbitals and each of them can hold two electrons, so it can hold a total of six electrons. 134k views 3 years ago. This type of hybridization is required. The carbon atoms of c2h2 are sp hybridized. This is the steric number (sn) of the central atom. Web count the number of lone pairs + the number of atoms that are directly attached to the central atom. Promote one s orbital electron to a half. In your drawing for part b, what kind. 134k views 3 years ago. Web steps to draw hybridized orbitals: The carbon atoms of c2h2 are sp hybridized. In this video, we use. This makes it a challenge to draw,. 134k views 3 years ago. In your drawing for part b, what kind. Web hybridization of an s orbital with all three p orbitals (p x, p y, and p z) results in four sp 3 hybrid orbitals. Web this organic chemistry video tutorial provides a basic introduction into valence bond theory and hybrid atomic orbitals. Web steps to draw hybrid orbital diagrams for a molecule. Promote one s orbital electron to a half. Read through the provided information, and sketch the lewis dot diagram of the provided compound. Web we can find the hybridization of an atom in a molecule by either looking at the types of bonds surrounding the atom or by calculating its steric number. This type of hybridization is required. Web actually, you can view it as there are three 2p orbitals and each of them can hold two electrons, so it can hold a total of six electrons in the 2p orbitals. In this video, we use. The carbon atoms of c2h2 are sp hybridized. Describe the hybrid orbitals used in the formation of bonding for each atom in some carbon containing compounds. Let me draw them for. This is the steric number (sn) of the central atom. Web steps to draw hybridized orbitals: It explains how to find the hybridization of. Web so let me draw what it would look like, or our best visual, or our best ability to kind of conceptualize what the orbitals around the carbon might look like. In your drawing for part b, what kind.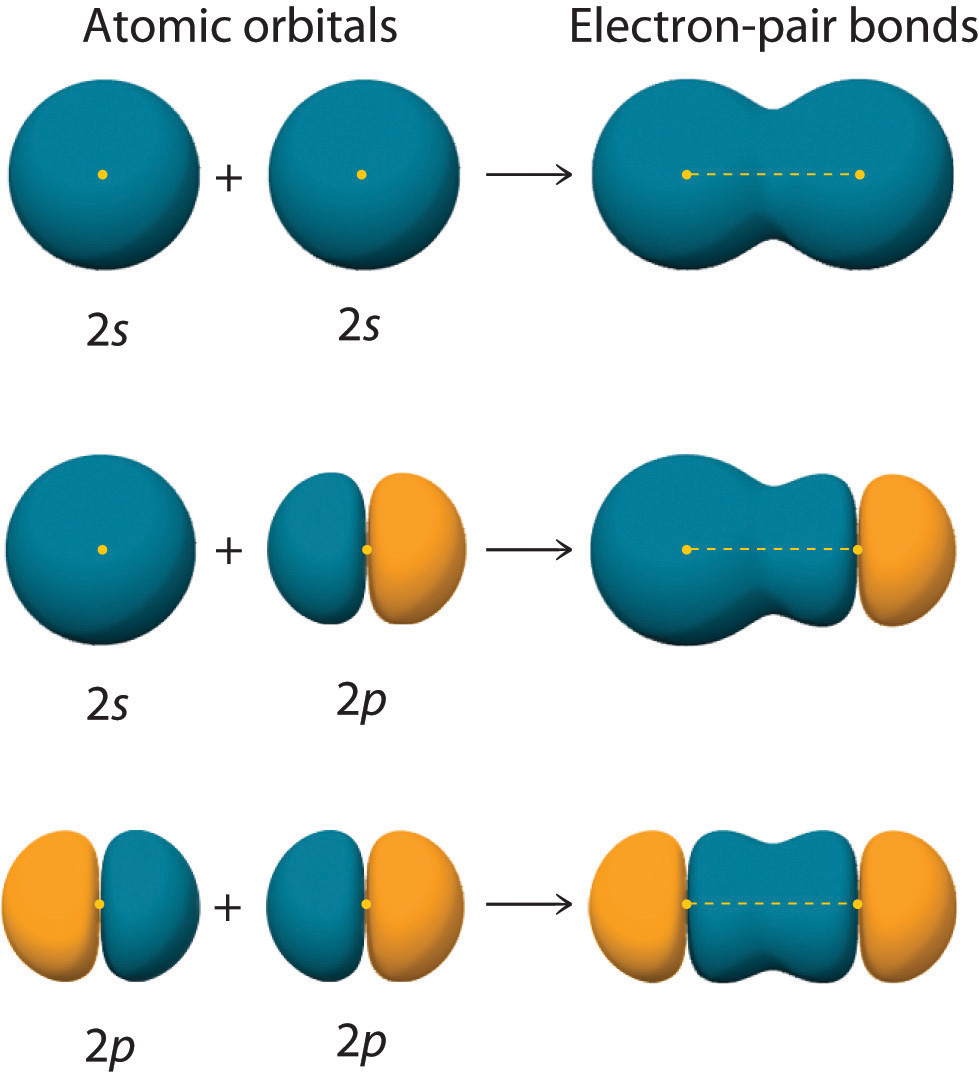
Chapter 6.2 Hybrid Orbitals Chemistry LibreTexts
Hybridization Definition, Types, Rules, Examples
2.2 Hybrid orbitals Chemistry LibreTexts
9.5 Hybrid Orbitals Chemistry LibreTexts
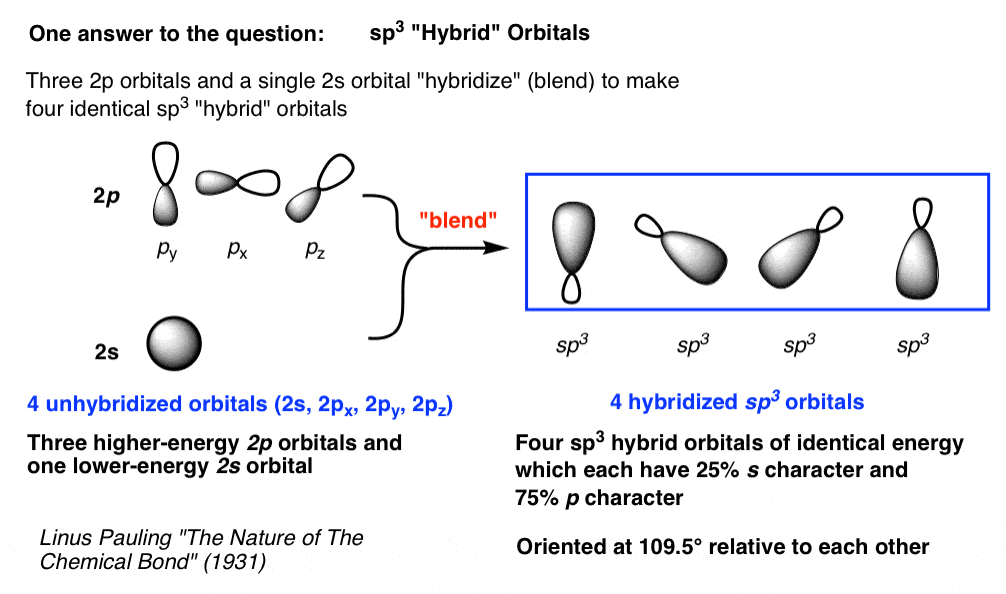
How To Draw Hybrid Orbitals » Doubleprogram
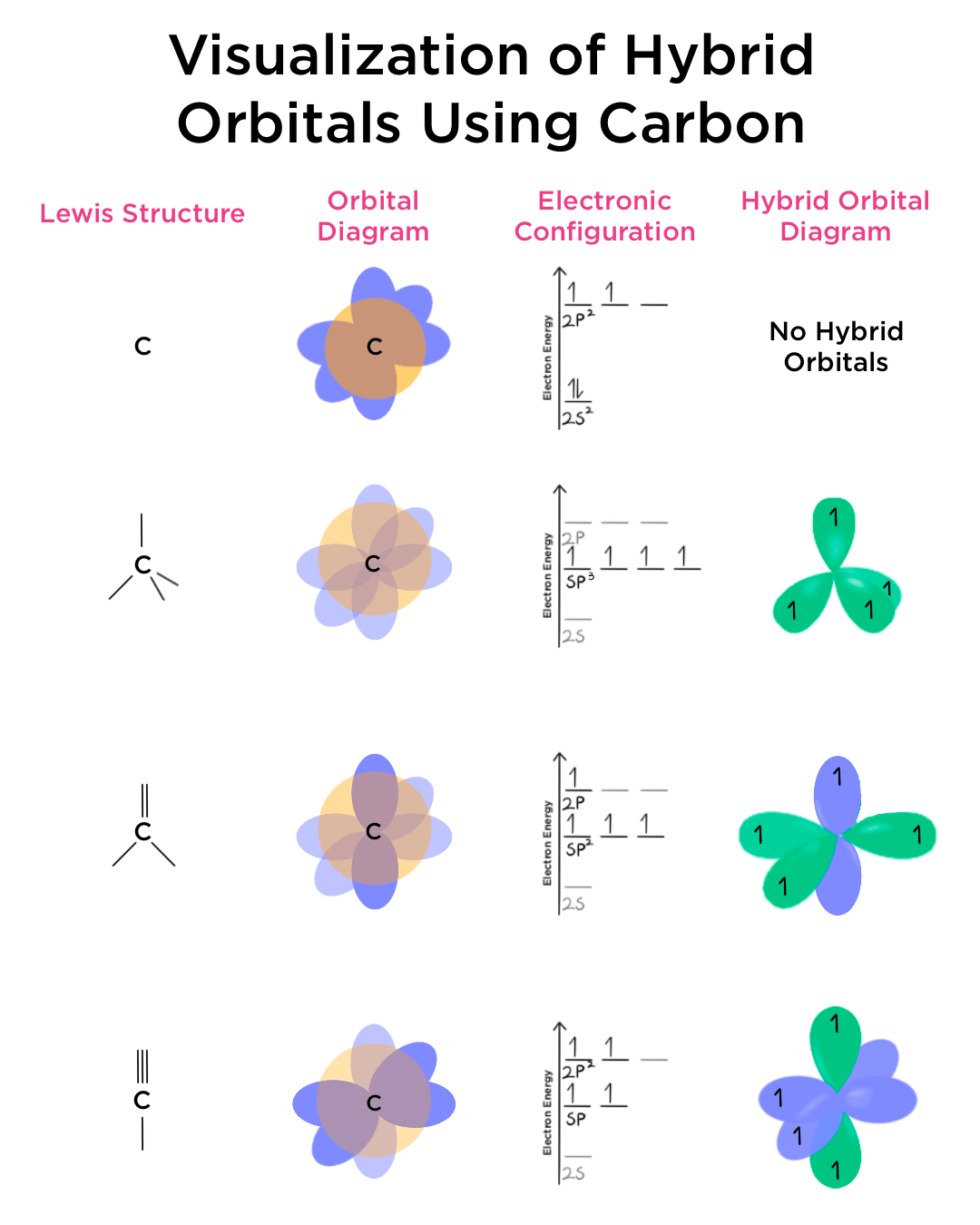
How To Draw Hybrid Orbitals Doubleprogram vrogue.co

Hybrid Atomic Orbitals Chemistry I
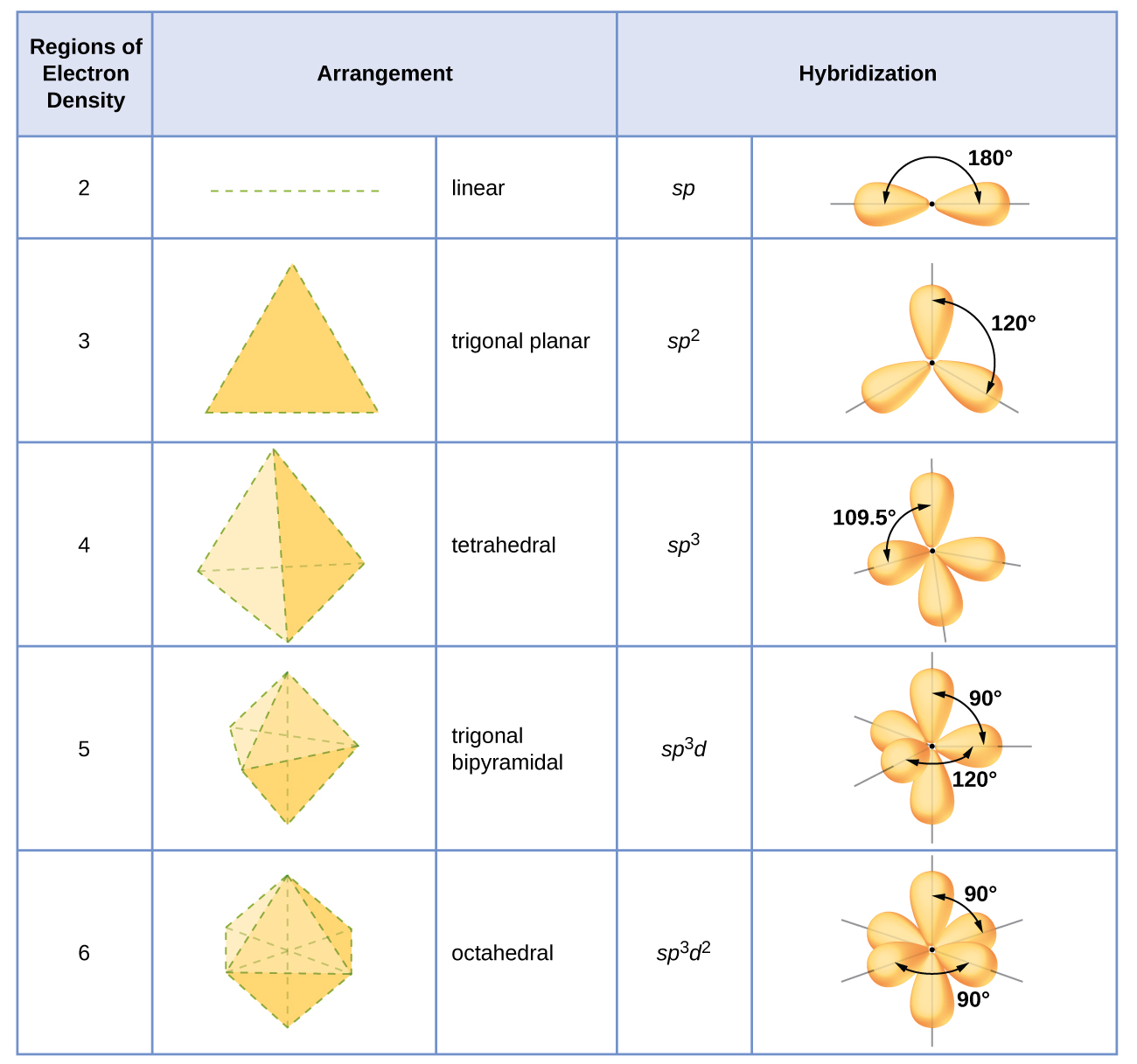
8.2 Hybrid Atomic Orbitals Chemistry LibreTexts
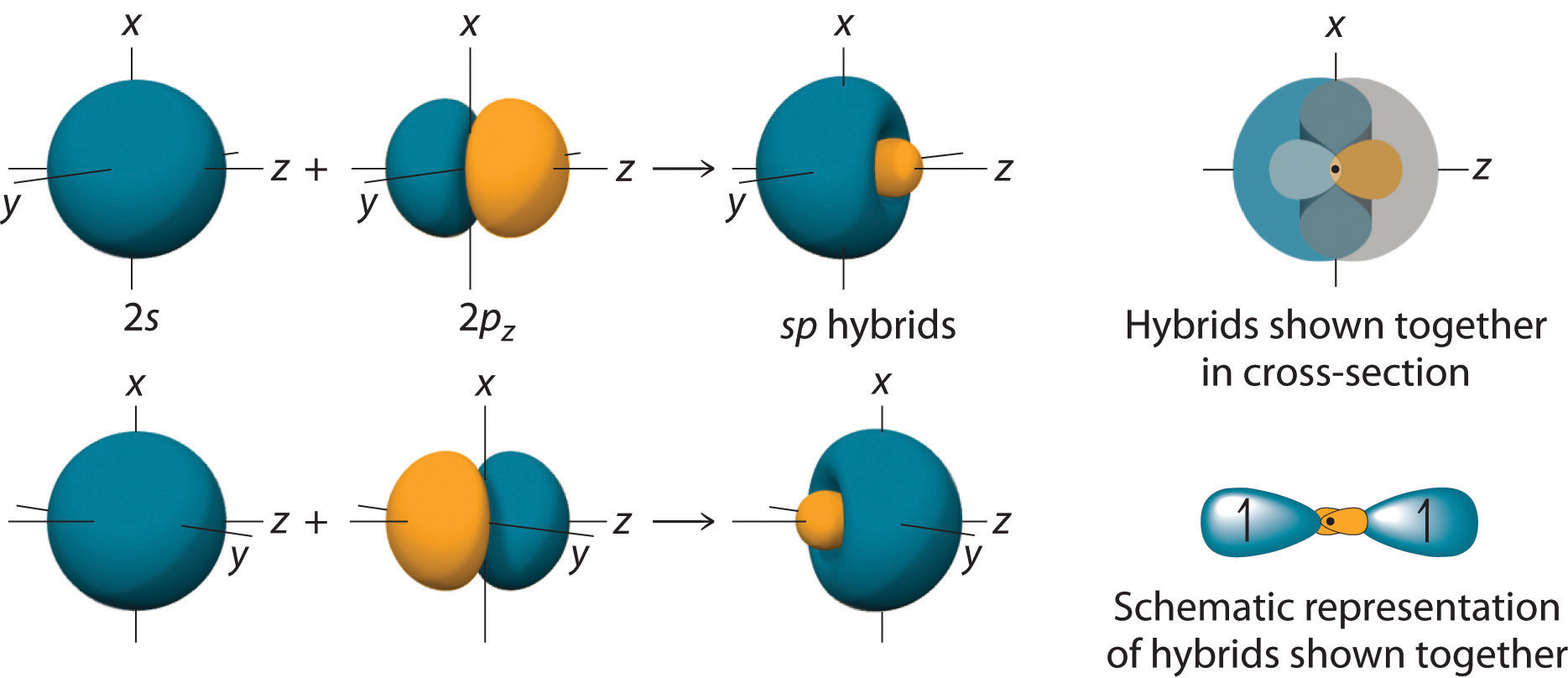
Localized Bonding and Hybrid Atomic Orbitals

How To Draw Hybrid Orbitals » Doubleprogram
Web Count The Number Of Lone Pairs + The Number Of Atoms That Are Directly Attached To The Central Atom.
Web In Sp Hybridization, One S Orbital And One P Orbital Hybridize To Form Two Sp Orbitals, Each Consisting Of 50% S Character And 50% P Character.
Draw A Figure Showing The Bonding Picture For The Imine Below.
So First I'll Draw The.
Related Post: