How To Draw Bohr Model
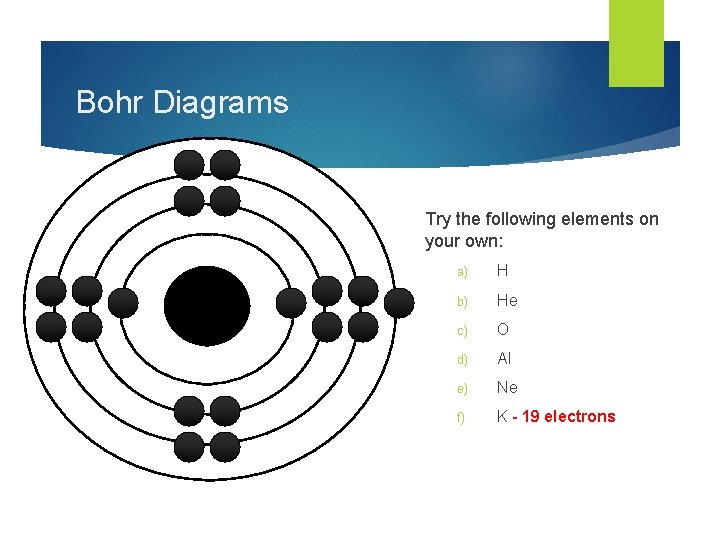
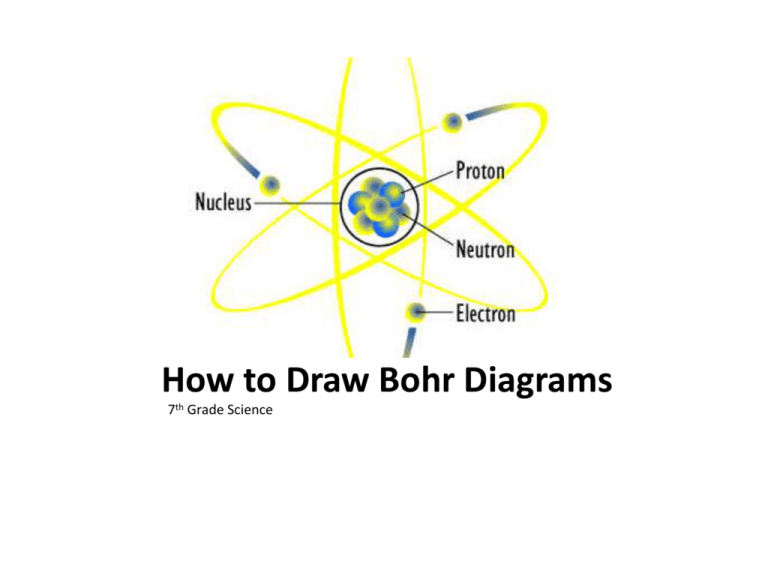
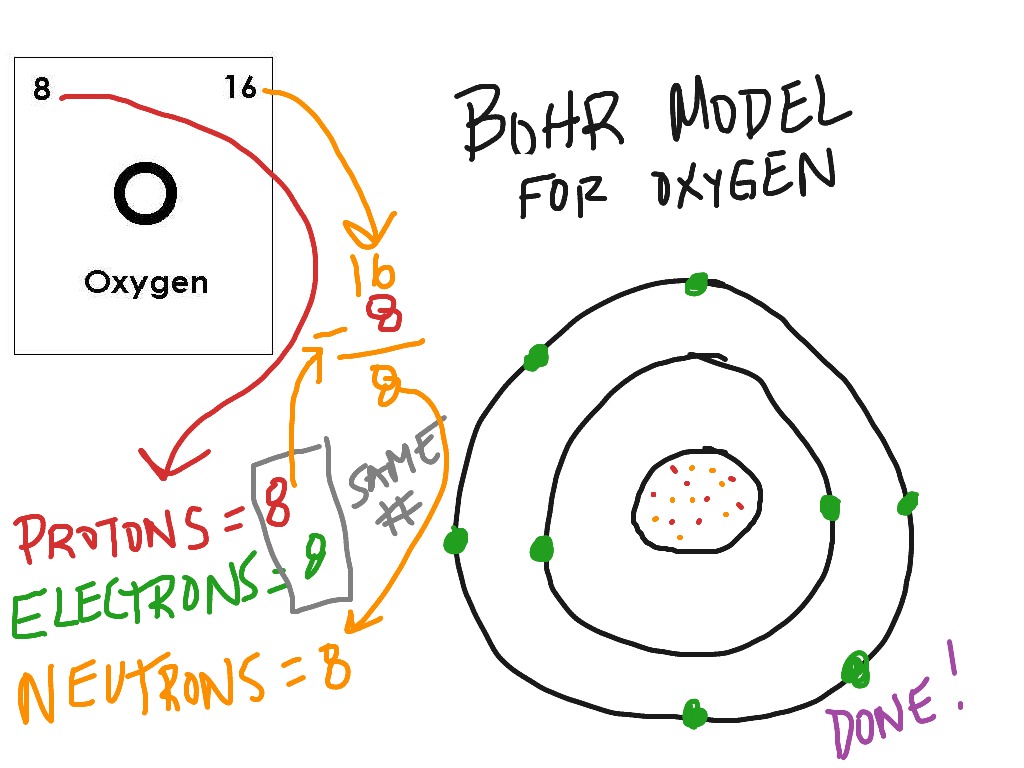
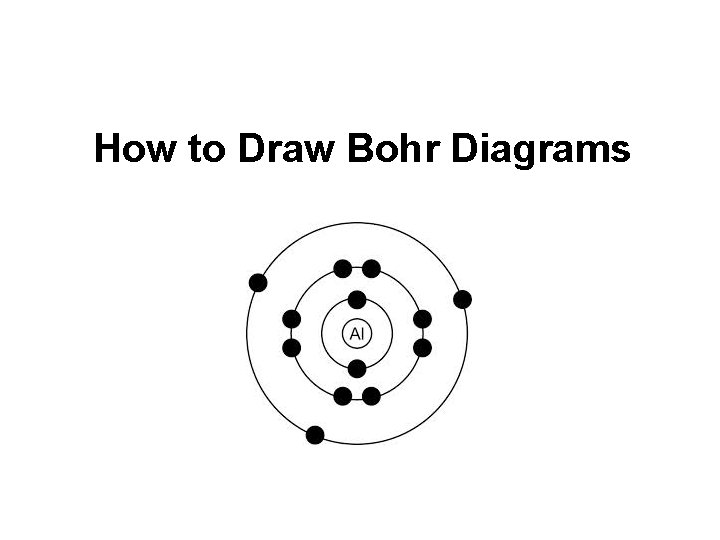
How To Draw Bohr Model - 4.7k views 2 years ago atomic structure. 1.3k views 1 year ago home school chemistry. But all of this is a lie…. Web drawing bohr models 1. Unfortunately, bohr could not explain why the electron should be restricted to particular orbits. Bohr's model calculated the following energies for an electron in the shell, n. Web bohr model, description of the structure of atoms proposed in 1913 by the danish physicist niels bohr. The following are his key contributions to our understanding of atomic structure: Web in the bohr model, there are a few rules that will help you draw accurate diagrams. Describe the arrangement of electrons using the shell model. Also, despite a great deal of tinkering, such as assuming. The ring closest to the nucleus is labelled n=1, the second ring from the nucleus is labelled n=2, and the third ring from the nucleus is labelled n=3. The highest energy form of electromagnetic waves are gamma (γ) rays and the lowest energy form are radio. But all of this. This video will show you how to draw bohr model of atom (or sometimes known as bohr diagram) correctly with. Use the rydberg equation to calculate energies of light emitted or absorbed by hydrogen atoms. The maximum number of electrons that can fill each shell is: From pn and rn, we can calculate the allowed energies from. Web to draw. These orbits form electron shells or energy levels, which are a way of visualizing the number of electrons in the various shells. But all of this is a lie…. Write the number of protons and neutrons at the center of the nucleus. Web a bohr model of a chlorine atom shows a nucleus surrounded by three concentric rings. Unfortunately, bohr. E ( n) = − 1 n 2 ⋅ 13.6 ev. Also, despite a great deal of tinkering, such as assuming. Draw the nucleus of an atom. 48k views 6 years ago science 10. Web this chemistry tutorial video walks you through how to draw a bohr model, bohr diagram, or planetary model for an atom or ion. Web discuss how the bohr model can be used to explain atomic spectra. Draw the bohr model for boron. Drawing a shell model diagram and an energy diagram for hydrogen, and then using the diagrams to calculate the energy required to excite an electron between different energy levels. E ( n) = − 1 n 2 ⋅ 13.6 ev. Web. Web bohr model, description of the structure of atoms proposed in 1913 by the danish physicist niels bohr. It does introduce several important features of all models used to describe the distribution of electrons in an atom. Bohr's model calculated the following energies for an electron in the shell, n. The ring closest to the nucleus is labelled n=1, the. But all of this is a lie…. Web bohr’s model of the hydrogen atom gave an exact explanation for its observed emission spectrum. Find the number of protons, electrons, and neutrons of an atom. Web bohr model energy levels (video) | khan academy. 40k views 2 years ago chemistry. Unfortunately, bohr could not explain why the electron should be restricted to particular orbits. Draw the first electron shell and put the electrons as a dot in it. The maximum number of electrons that can fill each shell is: Web chemistry homework in 3 minutes or less!in this video, josh helps you with your chemistry homework by solving a question. From pn and rn, we can calculate the allowed energies from. Electrons must occupy the lowest available shell, closest to the nucleus. The protons and neutrons are placed into the nucleus and the. The highest energy form of electromagnetic waves are gamma (γ) rays and the lowest energy form are radio. But all of this is a lie…. Web how did bohr respond to the drawbacks in the rutherford model? Web to draw the bohr model of an atom, follow these basic steps. Electromagnetic waves have an extremely wide range of wavelengths, frequencies, and energies. Web bohr’s model of the hydrogen atom gave an exact explanation for its observed emission spectrum. Web bohr model, description of the structure. Describe the arrangement of electrons using the shell model. Intro to general chemistry 3h 51m. Web the bohr model shows the atom as a central nucleus containing protons and neutrons with the electrons in circular orbitals at specific distances from the nucleus (figure \(\pageindex{1}\)). Drawing a shell model diagram and an energy diagram for hydrogen, and then using the diagrams to calculate the energy required to excite an electron between different energy levels. Use the rydberg equation to calculate energies of light emitted or absorbed by hydrogen atoms. The maximum number of electrons that can fill each shell is: 415k views 12 years ago. Web how to draw bohr models. Home school chemistry day 15 unit 2: This video will show you how to draw bohr model of atom (or sometimes known as bohr diagram) correctly with. Web to draw the bohr model of an atom, follow these basic steps. Web discuss how the bohr model can be used to explain atomic spectra. Calculating electron energy for levels n=1 to 3. Two in the first shell, eight in the second shell, eight in the third shell. Write the number of protons and neutrons in the nucleus p:9 n:10. Web a bohr model of a chlorine atom shows a nucleus surrounded by three concentric rings.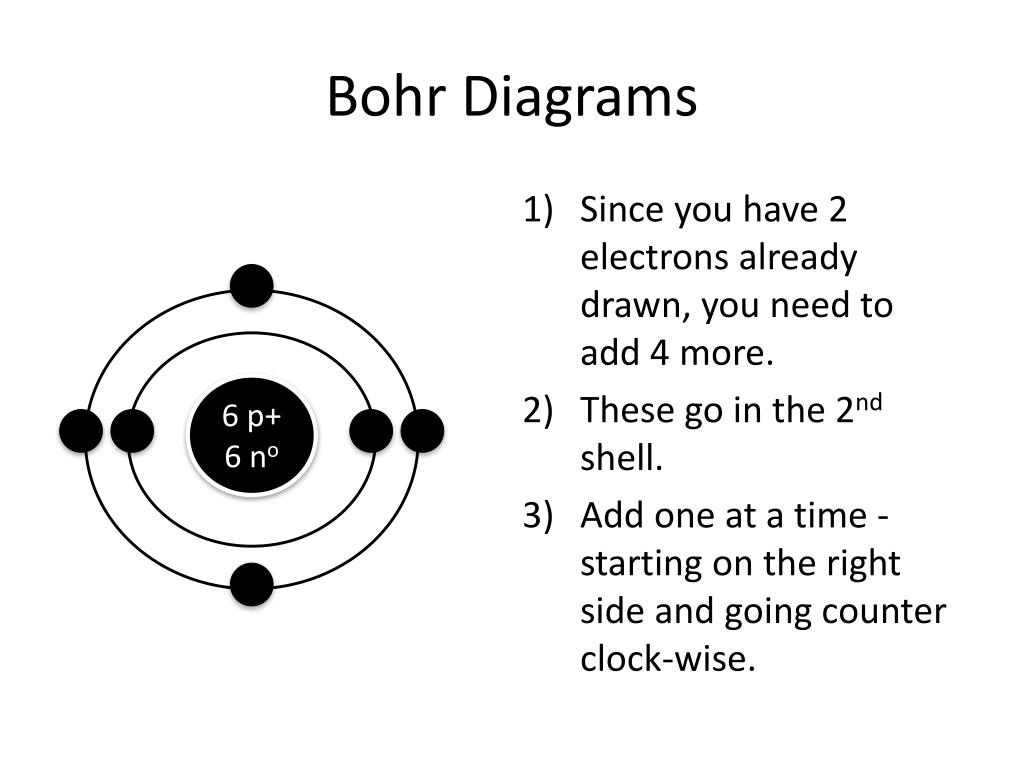
PPT How to Draw Bohr Diagrams PowerPoint Presentation, free download
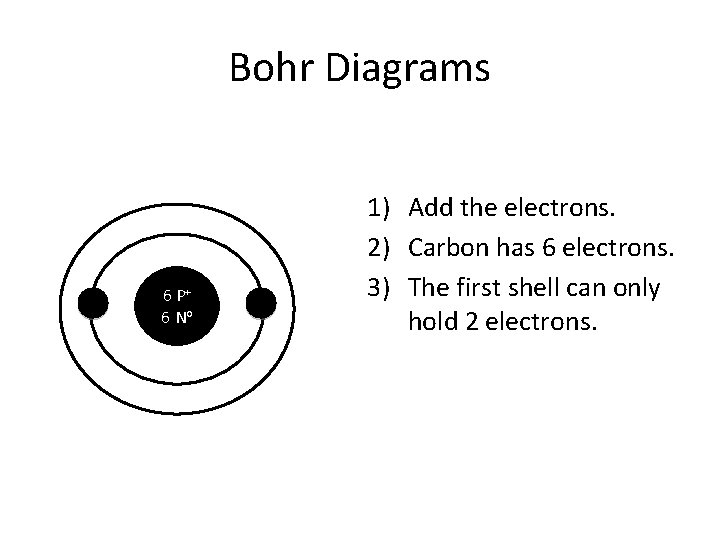
How to Draw Bohr Diagrams Bohr Diagrams 1
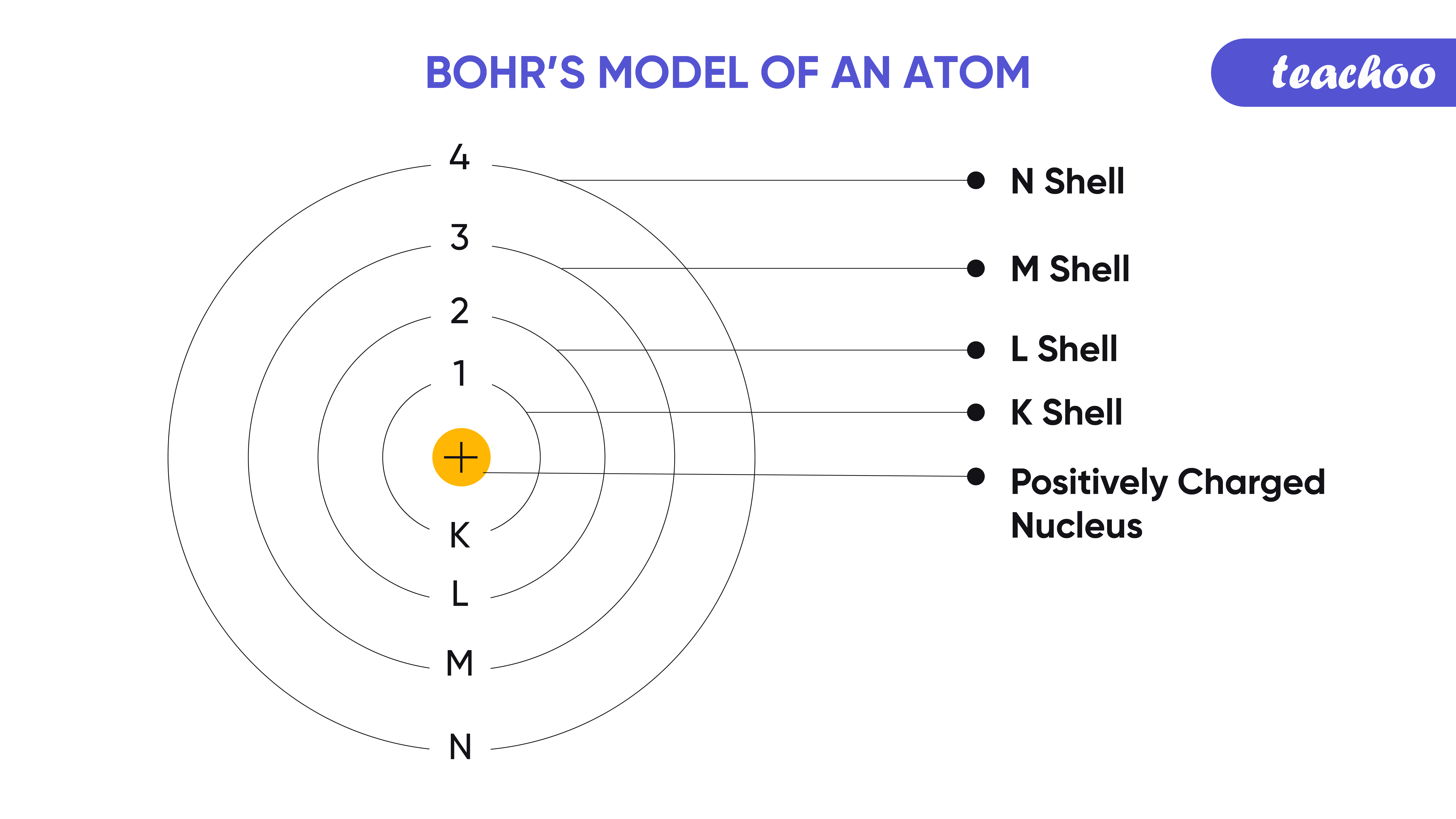
Bohr's Model of Atom Postulate and it's limitations Teachoo
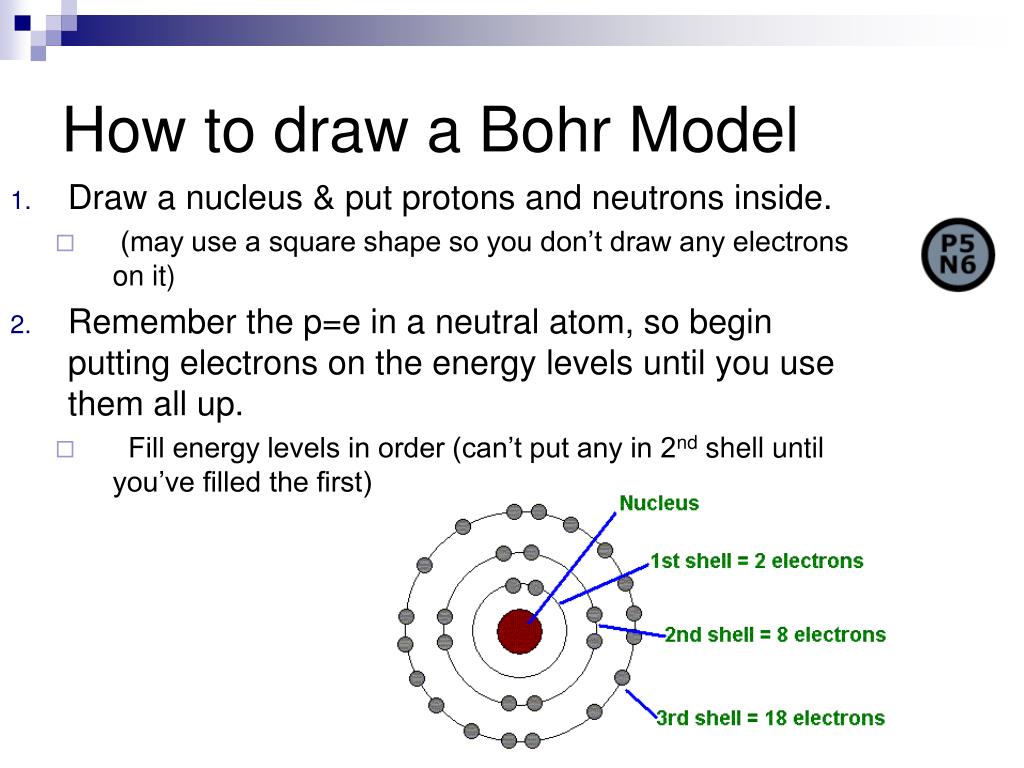
PPT Intro to Chemistry PowerPoint Presentation, free download ID
How to Draw Bohr Diagrams Bohr Diagrams 1
How to Draw Bohr Diagrams
how to draw a bohr model
How to Draw Bohr Diagrams Bohr Diagrams 1

Boron Bohr Model How To Draw Bohr Diagram For Boron B Atom Riset
PPT How to Draw Bohr Diagrams PowerPoint Presentation, free download
Draw The First Electron Shell And Put The Electrons As A Dot In It.
Rules For Energy Levels Keep Adding Shells Until You Use Up All Of Your Electrons.
Web Thus, \Begin {Align*}P_N R_N &=N\Hbar \\ [4Pt] P_N &=\Dfrac {N\Hbar} {R_N}\\ [4Pt] &=\Dfrac {\Hbar Z} {A_0 N} \\ [4Pt] &= \Dfrac {Ze^2 M_E} {4\Pi \Epsilon_0 \Hbar N}\End {Align*.
Web Bohr’s Model Of The Hydrogen Atom Gave An Exact Explanation For Its Observed Emission Spectrum.
Related Post: