How To Draw A Hydrogen Atom
How To Draw A Hydrogen Atom - Each nitrogen atom has three bonds. Bohr's model does not work for systems with more than one electron. Web the hydrogen atom is the simplest atom in nature and, therefore, a good starting point to study atoms and atomic structure. Web figure 2.2.1 2.2. Hydrogen is the main component of stars, and a star is,. Each carbon atom has four bonds. Web for example, the lewis electron dot diagram for hydrogen is simply \[\mathbf{h}\mathbf{\cdot}\nonumber \] because the side is not important, the lewis electron dot diagram could also be drawn as follows: 14k views 1 year ago. Web imagine shells around the nucleus, that get bigger and bigger. Web energy level diagrams and the hydrogen atom. Hydrogen is the main component of stars, and a star is,. Write protons, neutrons, and electrons of hydrogen atom. Web the hydrogen atom is the simplest atom in nature and, therefore, a good starting point to study atoms and atomic structure. The structure of the atom. Identify the physical significance of each of the quantum numbers ( n, l, m). Web when drawing the structure of a neutral organic compound, you will find it helpful to remember that. Web the hydrogen atom is the simplest atom in nature and, therefore, a good starting point to study atoms and atomic structure. It's the one with the lowest energy. I’ve created an interactive app that will draw atoms (of the first 20. Practice with drawing lewis structures. The hydrogen atom consists of a single negatively charged electron that moves about a positively charged proton ( figure 8.2 ). Describe the hydrogen atom in terms of wave function, probability density, total energy, and orbital angular momentum. Web bohr explained the hydrogen spectrum in terms of electrons absorbing and emitting photons to change energy. Try out different models by shooting light at the atom. Web a single hydrogen atom has one valence electron, so it only needs to react with one other atom covalently with a single bond to get a second electron. Web drawing a shell model diagram and an energy diagram for hydrogen, and then using the diagrams to calculate the energy. Atoms have protons and neutrons in the center, making the nucleus, while the electrons orbit the nucleus. Write protons, neutrons, and electrons of hydrogen atom. Web a single hydrogen atom has one valence electron, so it only needs to react with one other atom covalently with a single bond to get a second electron. Hydrogen is the main component of. Web to draw the lewis structure of an atom, write the symbol of the atom and draw dots around it to represent the valence electrons. The n = 1 state is known as the ground state, while higher n states are known as excited states. For the h+ structure use the periodic table to find the total. If the electron. If the electron in the atom makes a transition from. We’ll use a bohr diagram to. 5.5k views 8 years ago. Check how the prediction of the model matches the experimental results. The nucleus of a hydrogen atom contains 1 proton and 0 neutron. For the h2 structure use the periodic table to find the total number of. Now let's have a look at each shell in. Hydrogen is the main component of stars, and a star is,. Bohr's model does not work for systems with more than one electron. Try out different models by shooting light at the atom. Then comes shell number 2, and so on. Not even under a complex microscopic can we view the individual electrons that surround an atom’s nuclei. 5.5k views 8 years ago. So draw the nucleus of hydrogen atom as follows: 14k views 1 year ago. Draw nucleus of hydrogen atom. Each nitrogen atom has three bonds. For the h2 structure use the periodic table to find the total number of. 14k views 1 year ago. Web a single hydrogen atom has one valence electron, so it only needs to react with one other atom covalently with a single bond to get a second electron. Web energy level diagrams and the hydrogen atom. Now let's have a look at each shell in. The only thing smaller than atoms is their subatomic particles; Then comes shell number 2, and so on. 14k views 1 year ago. 5.5k views 8 years ago. The smallest, nearest to the nucleus is shell number 1. Practice with drawing lewis structures. By the end of this section, you will be able to: Hydrogen has 1 proton, 0 neutron, and 1 electron. The modern atomic theory states that atoms of one element are the same, while atoms of different elements are different. Web for example, the lewis electron dot diagram for hydrogen is simply \[\mathbf{h}\mathbf{\cdot}\nonumber \] because the side is not important, the lewis electron dot diagram could also be drawn as follows: Each nitrogen atom has three bonds. So draw the nucleus of hydrogen atom as follows: The diagram for hydrogen is shown above. Bohr's model does not work for systems with more than one electron.
Hydrogen Atomic Structure

Describe Bohr’s model of the hydrogen atom. bitWise Academy
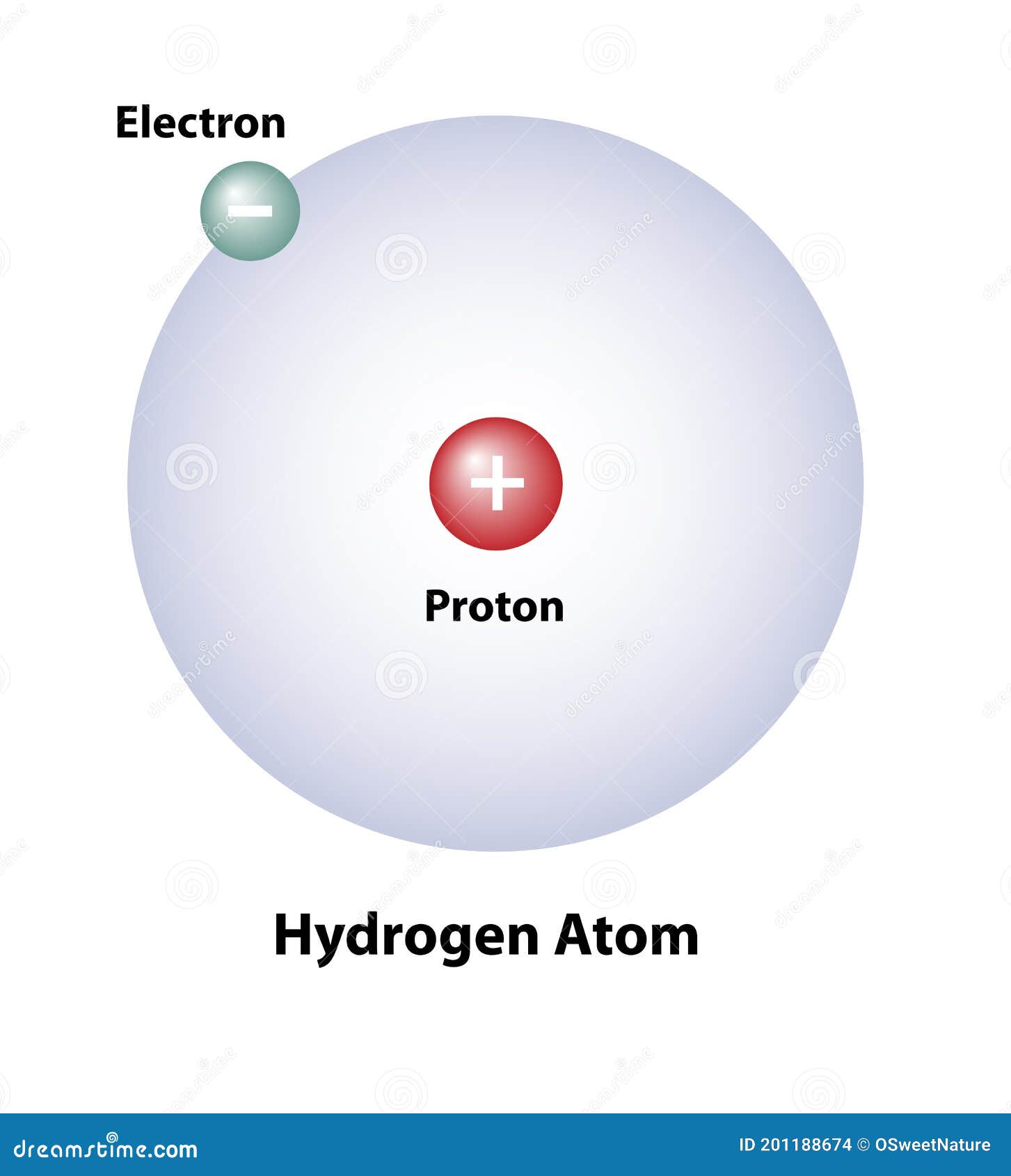
Molecular Structure of a Hydrogen Atom Stock Vector Illustration of

Diagram representation element hydrogen Royalty Free Vector

Diagram Representation Of The Element Hydrogen Stock Vector Image
Atom Drawing How To Draw An Atom Step By Step
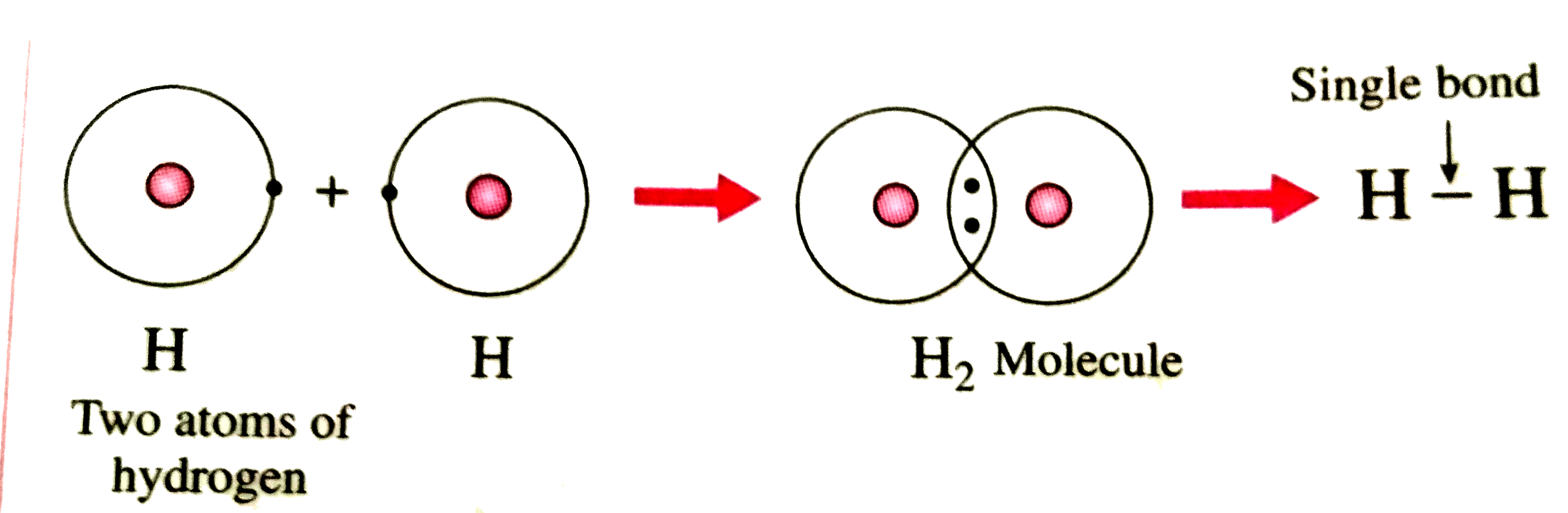
How is hydrogen molecule formed
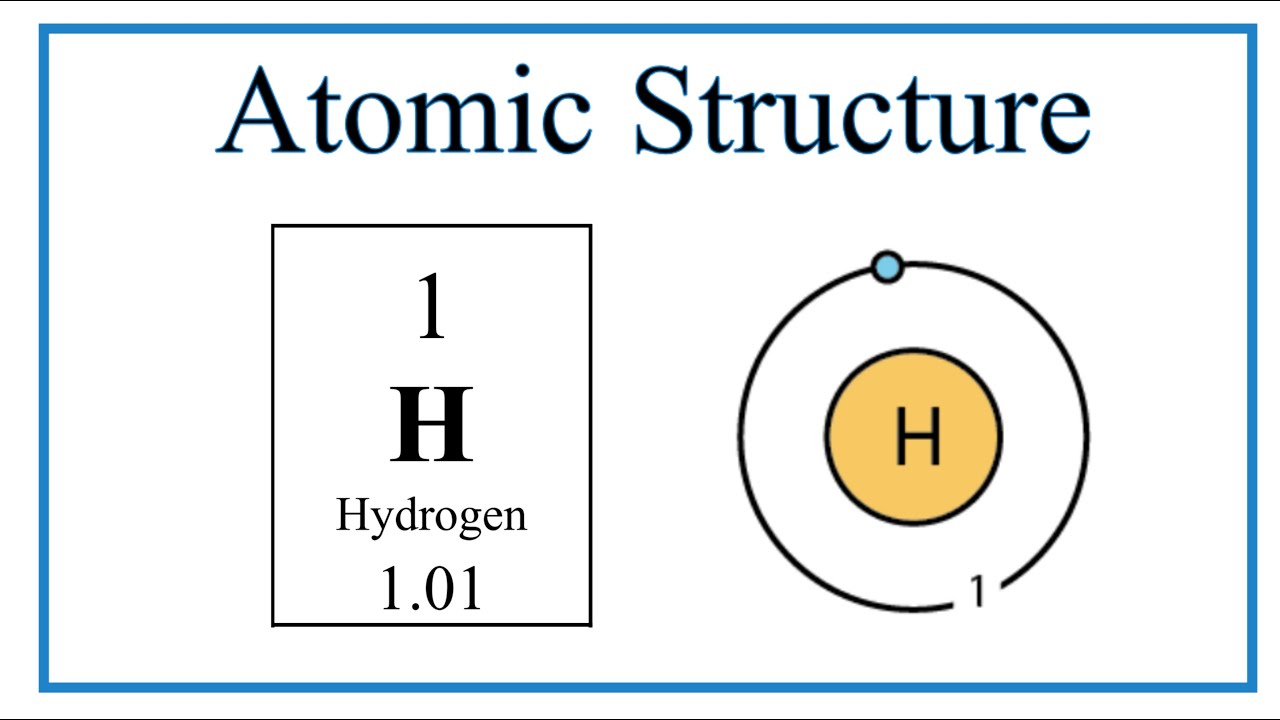
Atomic Structure (Bohr Model) for Hydrogen (H) YouTube

Hydrogen atom diagram concept illustration Stock Vector Image & Art Alamy

How to Learn About the Chemistry of the Hydrogen Atom 12 Steps
Web The Hydrogen Atom Is The Simplest Atom In Nature And, Therefore, A Good Starting Point To Study Atoms And Atomic Structure.
Web When Drawing The Structure Of A Neutral Organic Compound, You Will Find It Helpful To Remember That.
Web How Did Scientists Figure Out The Structure Of Atoms Without Looking At Them?
Each Hydrogen Atom Has One Bond.
Related Post: