How Do You Draw An Ionic Bond
How Do You Draw An Ionic Bond - Web the two ions each have octets as their valence shell, and the two oppositely charged particles attract, making an ionic bond: Web one type of chemical bond is an ionic bond. Web an ionic bond, also known as an electrovalent bond, is a type of chemical bond formed due to the electrostatic attraction between oppositely charged ions in a compound or molecule. Ionic bond forms when the valence (outermost) electrons of one atom are transferred permanently to another atom following the octet rule. 5.2k views 8 years ago chemical bonding. Look the metal has no valence electrons and the nonmetal is full. This crash course chemistry video tutorial explains the main concepts between ionic bonds found in. In a covalent bond, the electron is shared. Web in ionic bonding, atoms transfer electrons to each other. Web the two ions each have octets as their valence shell, and the two oppositely charged particles attract, making an ionic bond: Web 166k views 11 years ago chemical equations; When you draw an ion, don't forget [ ] and a charge. Ionic bonds require at least one electron donor and one electron acceptor. Ionic and covalent bonds are the two main types of chemical bonding. Web ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in. The oppositely charged ions are strongly attracted to each other, forming. They both achieve a more stable. Web the two ions each have octets as their valence shell, and the two oppositely charged particles attract, making an ionic bond: Magnesium has two electrons in its outer shell, oxygen has six. Ionic bonds result from the attraction between oppositely charged ions. Web the two ions each have octets as their valence shell, and the two oppositely charged particles attract, making an ionic bond: This crash course chemistry video tutorial explains the main concepts between ionic bonds found in. While you are learning how to draw dot and cross diagrams it’s useful to start with something you are already familiar with: Finally,. In contrast, atoms with the same electronegativity share electrons in covalent bonds, because neither atom preferentially attracts or repels the shared electrons. Web draw the electron configuration diagram for each atom. During ionic bonding the atoms form ions by gaining or losing electrons to obtain a full outer shell. Look the metal has no valence electrons and the nonmetal is. Electrostatics explains why this happens: A look at ionic bonding, where positive and. 298k views 3 years ago new ap & general chemistry video playlist. These oppositely charged ions attract each other to form ionic networks (or lattices). Draw the electron configuration diagrams for each atom in the compound. The main difference between ionic and covalent bonds is how equally the electrons are shared between atoms in the bond. Web one type of chemical bond is an ionic bond. Web ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. 224k views 5 years ago. Electrostatics explains why this happens: When you draw an ion, don't forget [ ] and a charge. This crash course chemistry video tutorial explains the main concepts between ionic bonds found in. Opposite charges attract and like charges repel. The energy of the electrostatic attraction (e), a measure of the force’s strength, is inversely proportional to the internuclear distance. For example, sodium cations (positively charged. The two ions attract each other according to coulombic interactions. They both achieve a more stable. Electrostatics explains why this happens: 298k views 3 years ago new ap & general chemistry video playlist. Swap the crosses for dots in one of your diagrams. Ionic bond forms when the valence (outermost) electrons of one atom are transferred permanently to another atom following the octet rule. When drawing lewis dot structures for ionic compounds you need to follow a different set of rules than with lewis structures for. Web in ionic bonding, atoms transfer electrons to each other. Then, identify the anion and write down. 224k views 5 years ago. Web the two ions each have octets as their valence shell, and the two oppositely charged particles attract, making an ionic bond: The energy of the electrostatic attraction (e), a measure of the force’s strength, is inversely proportional to the internuclear distance. Swap the crosses for dots in one of your diagrams. Rsc.li/2whsi4f if you. Magnesium has two electrons in its outer shell, oxygen has six. Web in an ionic bond, an electron is donated. 1.9m views 7 years ago. A look at ionic bonding, where positive and. The oppositely charged ions are strongly attracted to each other, forming. 298k views 3 years ago new ap & general chemistry video playlist. Web the organic chemistry tutor. Finally, combine the two ions to form an electrically neutral compound. You can see a listing of all my videos at my website,. Look the metal has no valence electrons and the nonmetal is full. Swap the crosses for dots in one of your diagrams. In contrast, atoms with the same electronegativity share electrons in covalent bonds, because neither atom preferentially attracts or repels the shared electrons. Web ionic bonds are formed when positively and negatively charged ions are held together by electrostatic forces. Ionic bonds are formed between cations and anions. Web draw the electron configuration diagram for each atom. Look at the number of electrons on the outer shell of each atom.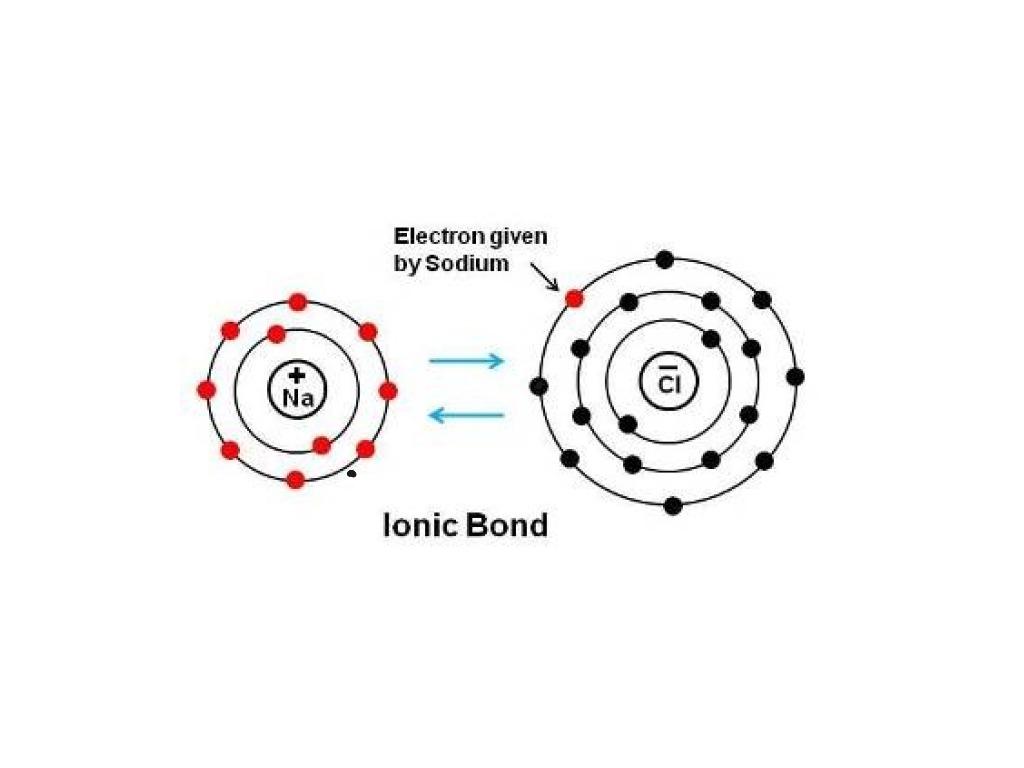
Ionic bond Science, Chemistry, Chemical Bonds ShowMe
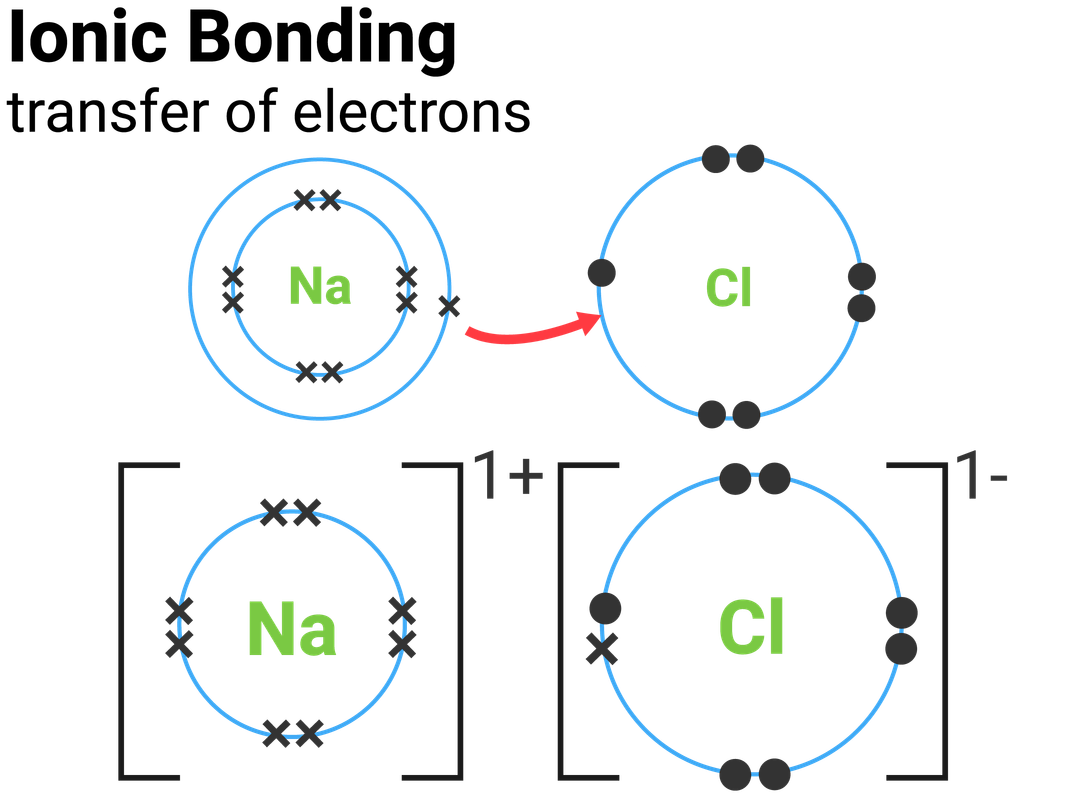
Chemical Bonds, Ionic, Covalent and Metallic AQA C2 revisechemistry.uk

Chemical Bonds, Ionic, Covalent and Metallic AQA C2 revisechemistry.uk
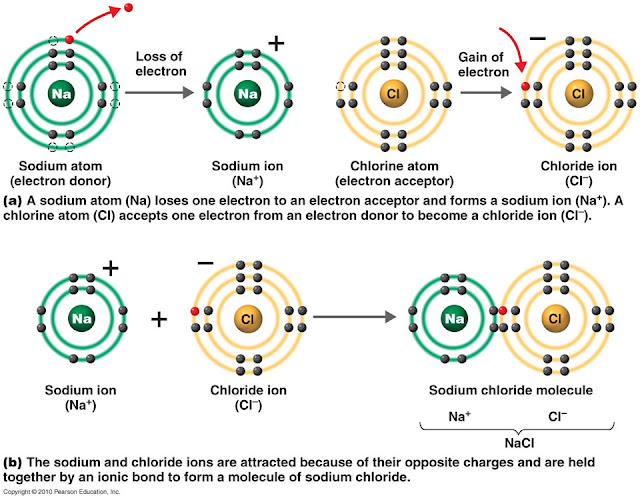
savvychemist Ionic Bonding (2) Dot and cross diagrams/Lewis structures

How Do Ions Form Ionic Bonds

chemical bonding Ionic and covalent compounds Britannica
Examples of Ionic Bonds and Compounds
Ionic Bonding Presentation Chemistry
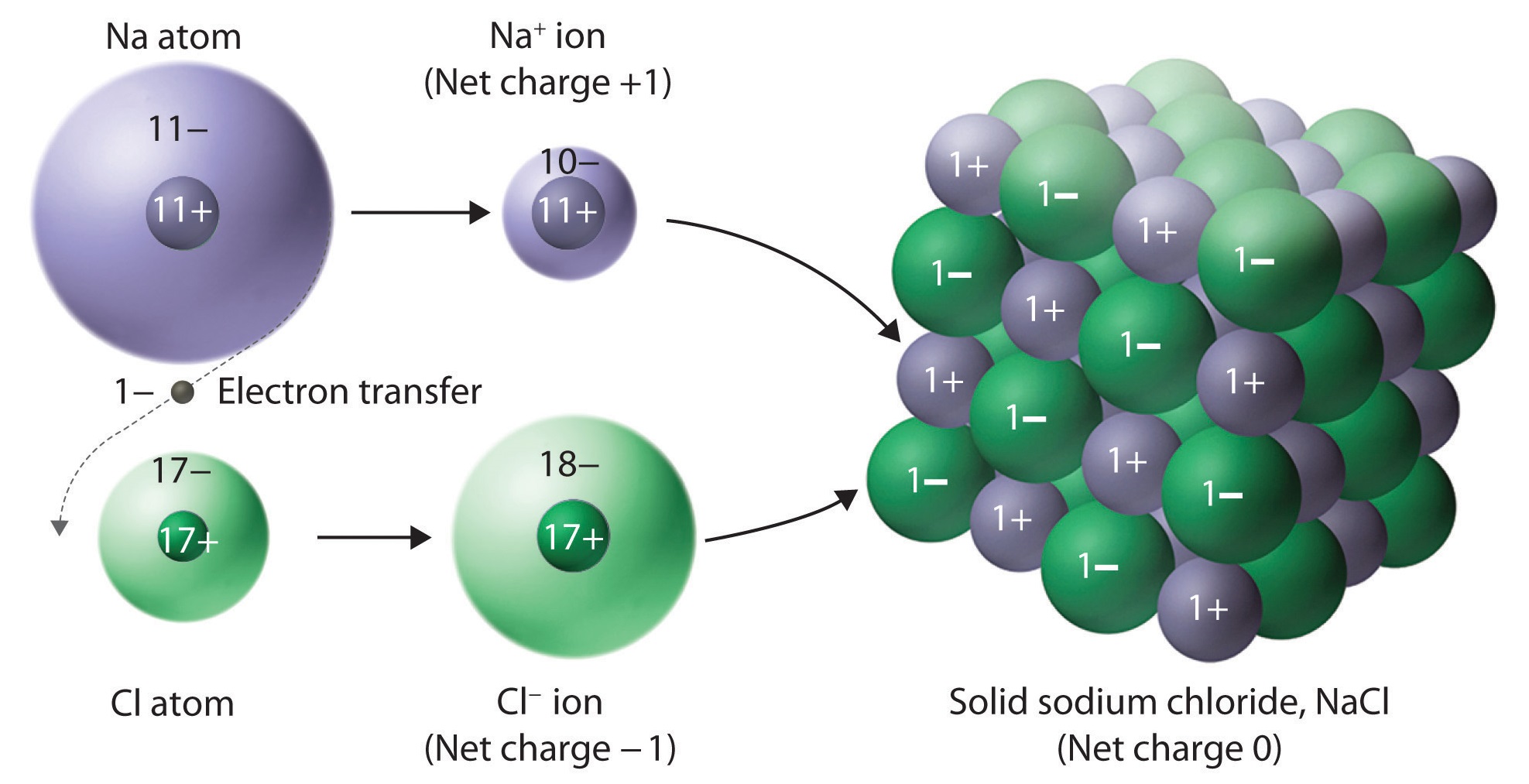
Ionic Solids Chemistry LibreTexts

ionic bond Definition, Properties, Examples, & Facts Britannica
Web The Two Ions Each Have Octets As Their Valence Shell, And The Two Oppositely Charged Particles Attract, Making An Ionic Bond:
During Ionic Bonding The Atoms Form Ions By Gaining Or Losing Electrons To Obtain A Full Outer Shell.
The Main Difference Between Ionic And Covalent Bonds Is How Equally The Electrons Are Shared Between Atoms In The Bond.
Ionic Bonds Require At Least One Electron Donor And One Electron Acceptor.
Related Post:
.PNG)