How Do You Draw An Ion
How Do You Draw An Ion - Use lewis diagrams to illustrate ion formation. Most atoms do not have eight electrons in their valence electron. Define the two types of ions. Web resulting in two ions—the na + ion and the cl − ion: For example, sodium cations (positively charged ions) and. Hydrogen is somewhat unusual in that it readily forms both cations and anions. 298k views 3 years ago new ap & general chemistry video playlist. This chemistry video explains how to draw the lewis structures of. Web common charges of ions formed by elements in different groups of the periodic table. Web draw a lewis electron dot diagram for an atom or a monatomic ion. Ions form when atoms lose or gain. As mentioned above, there are positive and negative ions. In almost all cases, chemical bonds are formed by interactions of valence electrons in atoms. We call these charged particles ions. Ionic bonds result from the attraction between oppositely charged ions. Web to find the electron configuration for an ion, first identify the configuration for the neutral atom. Use lewis diagrams to illustrate ion formation. 5.4k views 3 years ago chemistry (grade level, regents level) this chemistry tutorial video walks you through how to draw a bohr model, bohr diagram, or. A lewis diagram shows how the valence electrons are distributed. Or group of atoms with a positive or negative. Ionic bonds result from the attraction between oppositely charged ions. Most atoms do not have eight electrons in their valence electron. In almost all cases, chemical bonds are formed by interactions of valence electrons in atoms. Hydrogen is somewhat unusual in that it readily forms both cations and anions. Or group of atoms with a positive or negative. Most atoms do not have eight electrons in their valence electron. To obtain a full outer shell: Web they can gain a full outer shell by losing electrons and becoming positive or gaining electrons and becoming negative. Web one type of chemical bond is an ionic bond. Web draw a lewis electron dot diagram for an atom or a monatomic ion. Web during the formation of some compounds, atoms gain or lose electrons, and form electrically charged particles called ions (figure \(\pageindex{1}\)). To obtain a full outer shell: Web during ionic bonding the atoms form ions by gaining or losing electrons to obtain a full outer shell.. Most atoms do not have eight electrons in their valence electron. Web one type of chemical bond is an ionic bond. Ions form when atoms lose or gain. A lewis diagram shows how the valence electrons are distributed around the atoms in a molecule. Then, add or remove electrons depending on the ion's charge. For example, sodium cations (positively charged ions) and. Web they can gain a full outer shell by losing electrons and becoming positive or gaining electrons and becoming negative. Web one type of chemical bond is an ionic bond. What is the lewis electron dot symbol for each ion? Web during ionic bonding the atoms form ions by gaining or losing. Ions form when atoms lose or gain. Define the two types of ions. Web common charges of ions formed by elements in different groups of the periodic table. Ionic bonds result from the attraction between oppositely charged ions. Justify the observed charge of ions to their electronic configuration. To obtain a full outer shell: Web during ionic bonding the atoms form ions by gaining or losing electrons to obtain a full outer shell. Web during the formation of some compounds, atoms gain or lose electrons, and form electrically charged particles called ions (figure \(\pageindex{1}\)). 5.4k views 3 years ago chemistry (grade level, regents level) this chemistry tutorial video. Then, add or remove electrons depending on the ion's charge. Web during ionic bonding the atoms form ions by gaining or losing electrons to obtain a full outer shell. Ionic bonds result from the attraction between oppositely charged ions. Writing lewis dot symbols of ions. We call these charged particles ions. Use lewis diagrams to illustrate ion formation. Most atoms do not have eight electrons in their valence electron. 298k views 3 years ago new ap & general chemistry video playlist. Or group of atoms with a positive or negative. Web resulting in two ions—the na + ion and the cl − ion: Ionic bonds result from the attraction between oppositely charged ions. Web during the formation of some compounds, atoms gain or lose electrons, and form electrically charged particles called ions (figure \(\pageindex{1}\)). What is the lewis electron dot symbol for each ion? As mentioned above, there are positive and negative ions. Web to find the electron configuration for an ion, first identify the configuration for the neutral atom. Web common charges of ions formed by elements in different groups of the periodic table. We call these charged particles ions. In almost all cases, chemical bonds are formed by interactions of valence electrons in atoms. Then, add or remove electrons depending on the ion's charge. Web determine the electron configuration of ions. 5.4k views 3 years ago chemistry (grade level, regents level) this chemistry tutorial video walks you through how to draw a bohr model, bohr diagram, or.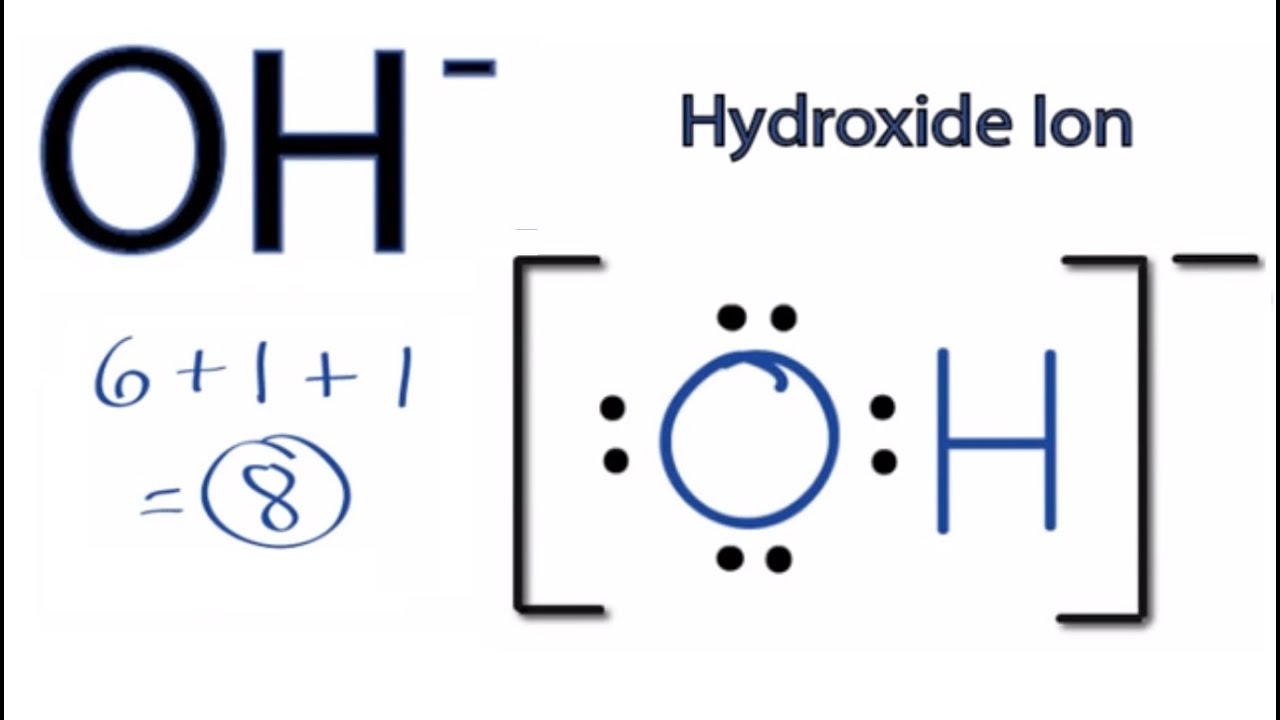
OH Lewis Structure How to Draw the Lewis Dot Structure for the

Formation of Ion SPM Chemistry

How to Draw an Ion YouTube

Drawing Ions Chemstuff
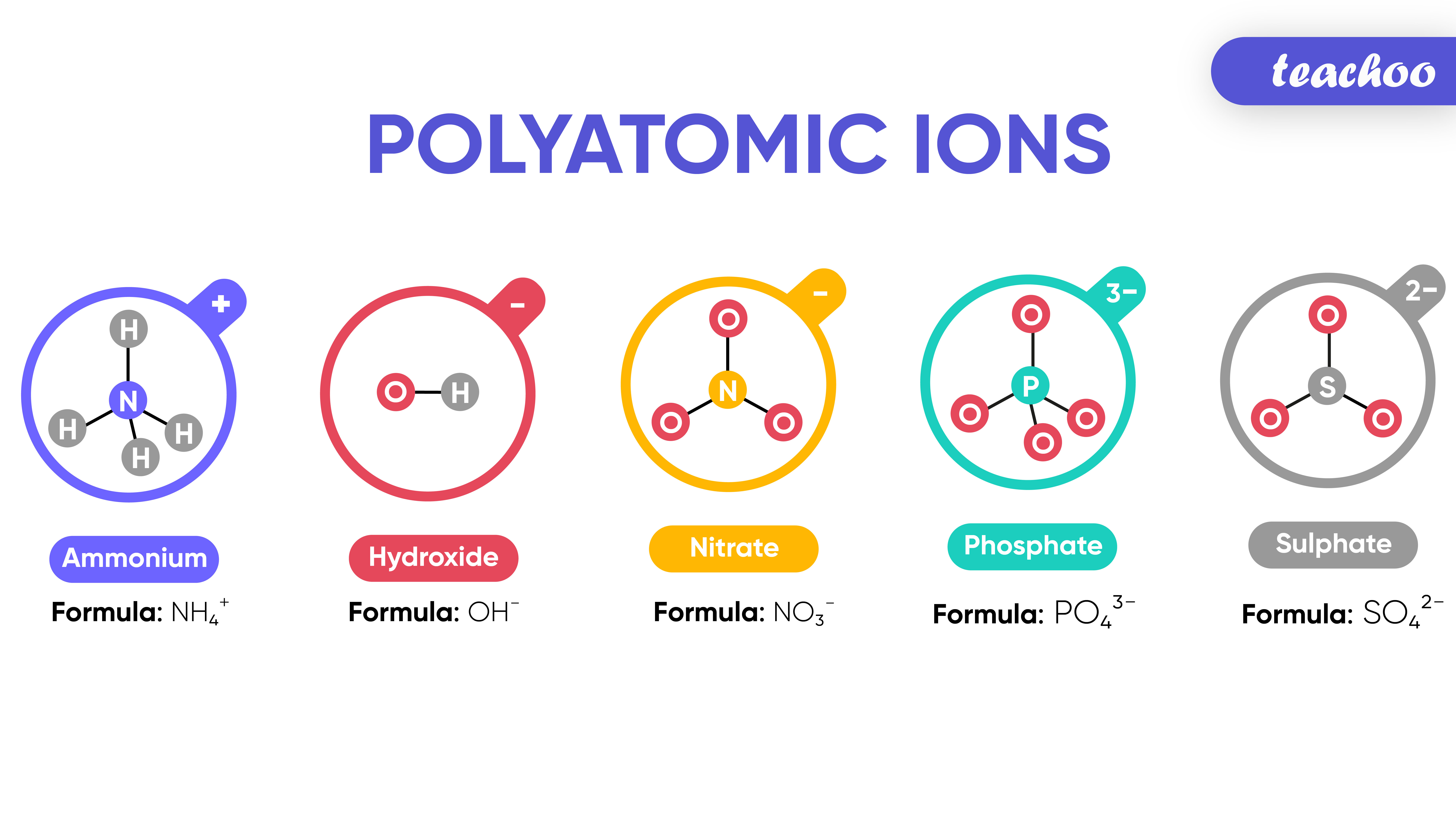
What are Polyatomic Ions? Give Examples Teachoo Concepts
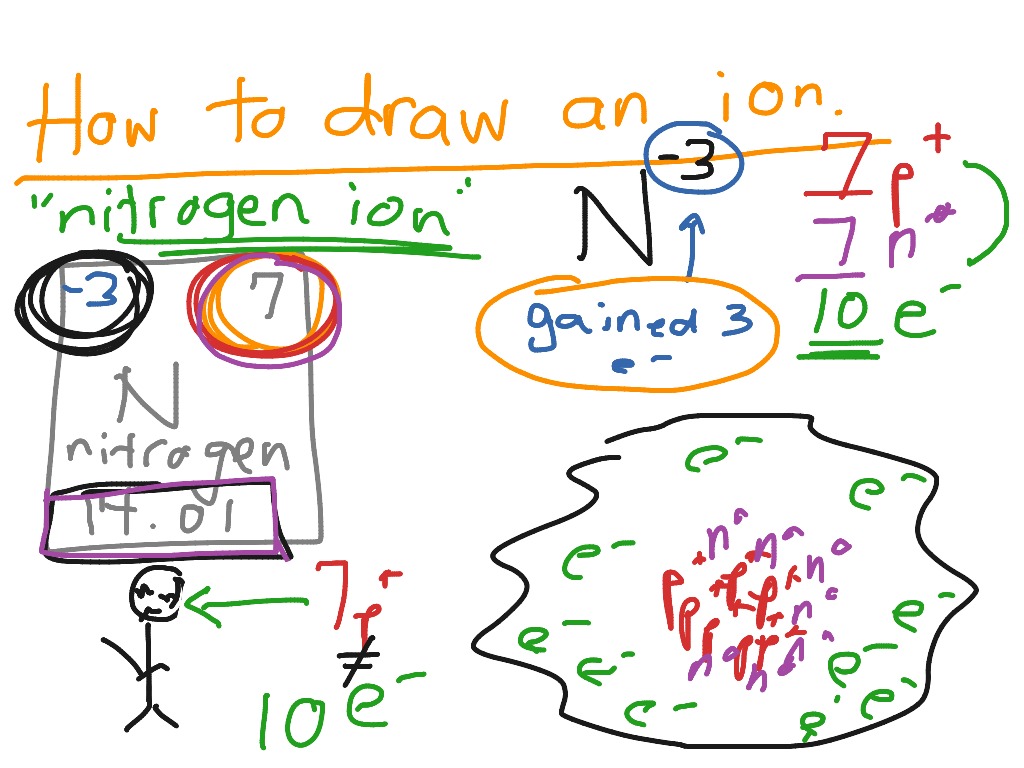
ShowMe how to draw an ion
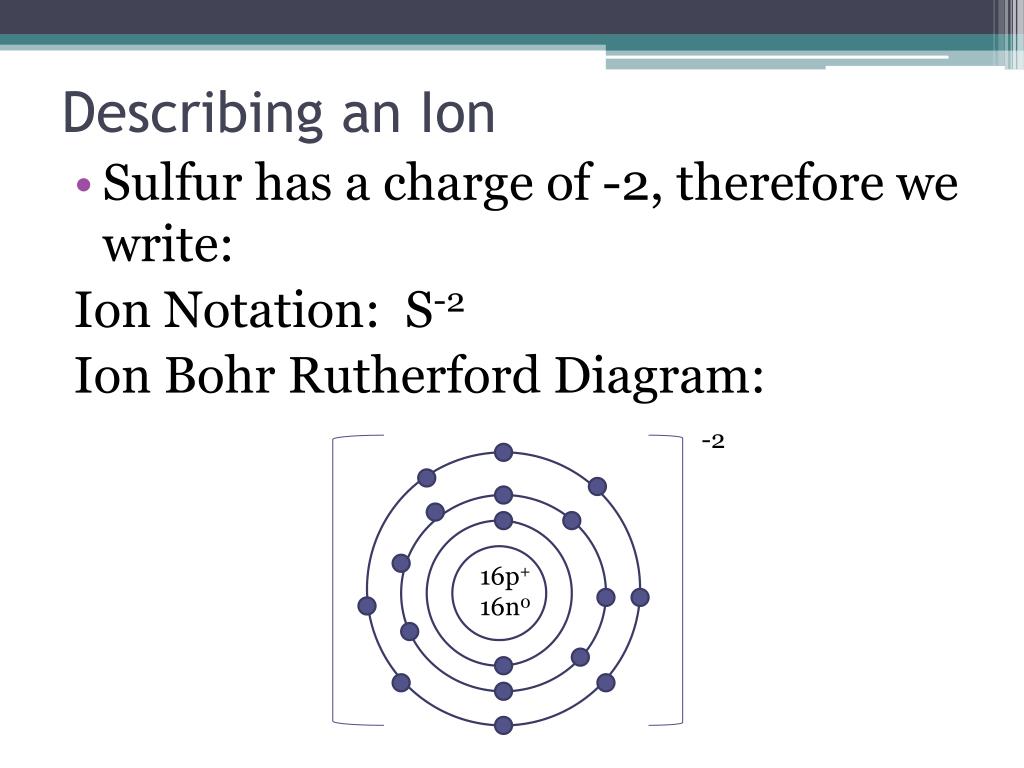
How To Draw A Bohr Model For An Ion
Ion atom molecule education poster Royalty Free Vector Image

Drawing Ions (NCEA L1 & Junior Science) YouTube

What are Ionic Compounds and how they are formed?
Magnesium Loses Two Electrons And Oxygen Gains Two Electrons,.
Justify The Observed Charge Of Ions To Their Electronic Configuration.
Writing Lewis Dot Symbols Of Ions.
A Lewis Diagram Shows How The Valence Electrons Are Distributed Around The Atoms In A Molecule.
Related Post: