How Do You Draw An Electron Dot Diagram
How Do You Draw An Electron Dot Diagram - Web electron dot diagrams are diagrams in which the valence electrons of an atom are shown as dots distributed around the element’s symbol. The diagram is also called a lewis dot diagram, lewis dot formula, or electron dot diagram. Web how to draw electron dot structures? Draw the atoms on paper and put dots around them to represent valence electrons of the atom. The first thing we would need to do is to find the total number of valence electrons. Web an electron configuration diagram is a model that depicts the position of electrons as they orbit the nucleus of an atom. Web its electron dot diagram is as follows: To facilitate our understanding of how valence electrons interact, a simple way of representing those valence electrons would be useful. More complicated versions can be used to show the bond between different atoms in a molecule. Web a lewis electron dot diagram (or electron dot diagram or a lewis diagram or a lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. Web a lewis structure is a diagram that shows the chemical bonds between atoms in a molecule and the valence electrons or lone pairs of electrons. Web to draw the lewis electron dot diagram we picture in our minds the symbol for mg in a box with all of its core electrons (i.e., 1 s2 2 s2 2 p6 ).. The diagram is also called a lewis dot diagram, lewis dot formula, or electron dot diagram. Stages to articulate the electron dot formula are stated beneath. Write the symbol of the atom you are drawing the electron dot diagram for in the middle of your paper. Then we place the valence electrons around the sides of the box with each. Web electron dot diagrams are diagrams in which the valence electrons of an atom are shown as dots distributed around the element’s symbol. These valence electrons are negatively charged and are attracted to the positively charged nucleus, made up of neutrons and protons. Web a lewis electron dot diagram (or electron dot diagram or a lewis diagram or a lewis. In almost all cases, chemical bonds are formed by interactions of valence electrons in atoms. Web to draw the lewis structure of an atom, write the symbol of the atom and draw dots around it to represent the valence electrons. Web draw a lewis electron dot diagram for any atom or a monatomic ion with an atomic number of less. In almost all cases, chemical bonds are formed by interactions of valence electrons in atoms. Web a lewis electron dot diagram (or electron dot diagram or a lewis diagram or a lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. How many valence electrons does magnesium have? To. Web an electron configuration diagram is a model that depicts the position of electrons as they orbit the nucleus of an atom. A lewis diagram shows how the valence electrons are distributed around the atoms in a molecule. Web a lewis electron dot diagram (or electron dot diagram or a lewis diagram or a lewis structure) is a representation of. Shared pairs of electrons are drawn as lines between atoms, while. The diagram is also called a lewis dot diagram, lewis dot formula, or electron dot diagram. Follow these simple steps to draw lewis dot structures: Web lewis structures (also known as lewis dot structures or electron dot structures) are diagrams that represent the valence electrons of atoms within a. These valence electrons are negatively charged and are attracted to the positively charged nucleus, made up of neutrons and protons. If the species is an ion, add or subtract electrons corresponding to the charge. Web here's some of the guidelines for drawing dot structures. You can alternate the dots and crosses in simple diagrams or use other colours or symbols. Follow these simple steps to draw lewis dot structures: Draw a lewis electron dot diagram for an atom or a monatomic ion. Web a video tutorial for how to draw lewis structures in five steps. A lewis electron dot formula comprises one dot for every valence electron and an element’s symbol. Web lewis dot structures, or lewis structures for short,. Web to draw the lewis structure of an atom, write the symbol of the atom and draw dots around it to represent the valence electrons. Since electrons repel each other, the dots for a given atom are distributed evenly around the symbol before they are paired. Web electron dot diagrams are diagrams in which the valence electrons of an atom. Web a lewis electron dot diagram (or electron dot diagram or a lewis diagram or a lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. So let's say we wanted to draw the dot structure for this molecule, so silicon tetrafluoride. Since electrons repel each other, the dots for a given atom are distributed evenly around the symbol before they are paired. Be sure to have the correct number of electrons. To facilitate our understanding of how valence electrons interact, a simple way of representing those valence electrons would be useful. How many valence electrons does magnesium have? More complicated versions can be used to show the bond between different atoms in a molecule. In almost all cases, chemical bonds are formed by interactions of valence electrons in atoms. You can alternate the dots and crosses in simple diagrams or use other colours or symbols for larger diagrams. Web here's some of the guidelines for drawing dot structures. Web lewis dot structures, or lewis structures for short, are visuals that represent the outermost shell of electrons, also known as valence electrons, and possible covalent bonds within an atom or molecule. Shared pairs of electrons are drawn as lines between atoms, while. The sum of the valence electrons is. A beryllium atom, with two valence electrons, has the electron dot diagram below: Here we have changed the electrons on the hydrogen atoms to be dots. Write the symbol of the atom you are drawing the electron dot diagram for in the middle of your paper.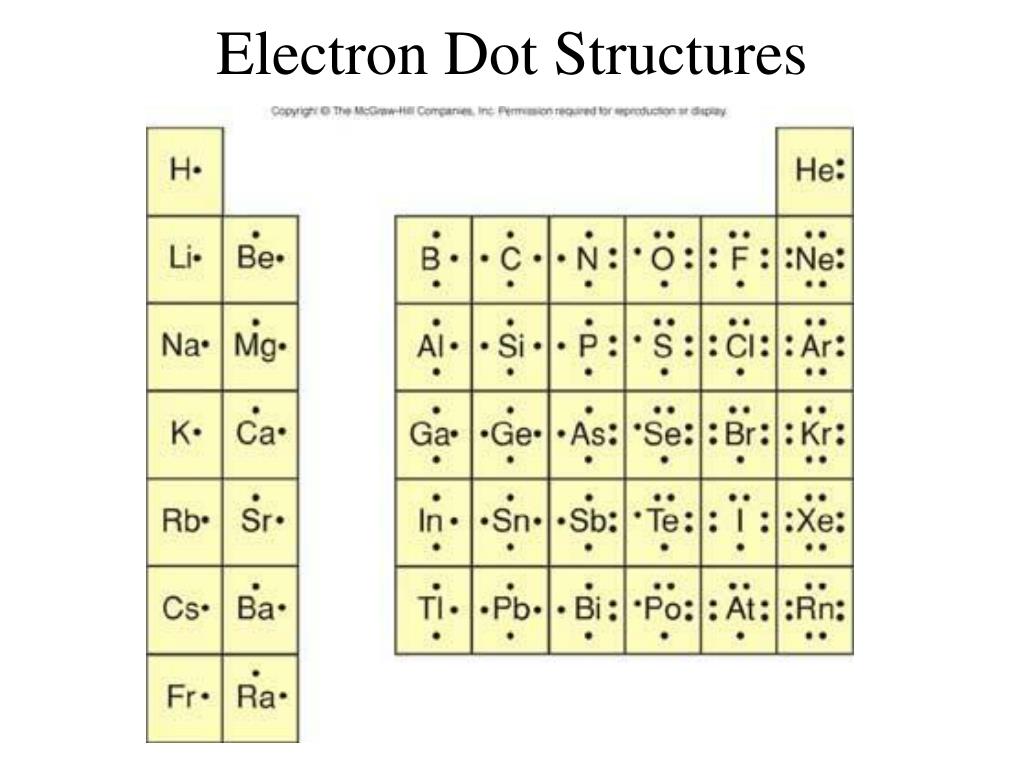
PPT Electron Dot Structures PowerPoint Presentation, free download

PPT Drawing Lewis Structures A Tutorial on Writing Lewis Dot

How to Draw a Lewis Structure

Lewis Dot Structure Definition, Examples, and Drawing

BohrRutherford Diagrams & Lewis Dot Diagrams Eve Wongworakul

3 Ways to Draw Lewis Dot Structures wikiHow

How To Draw Lewis Dot Diagrams
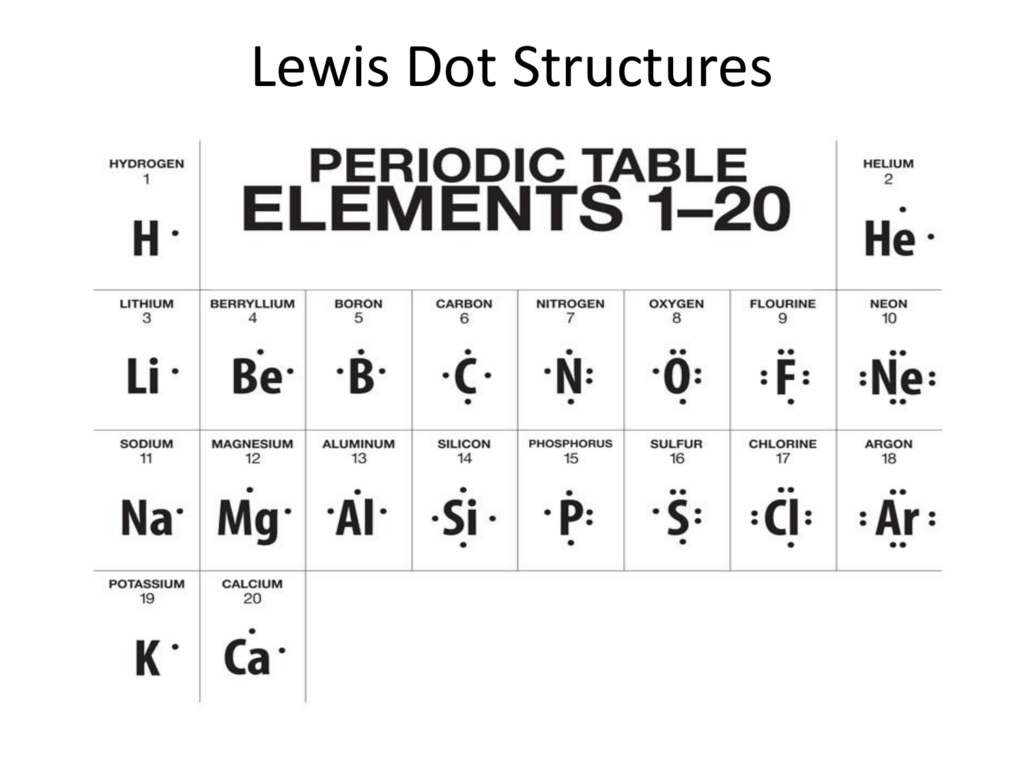
Lewis Dot Structures

How to Draw Electron Dot Diagrams Sciencing
Valence Electrons Presentation Chemistry
The Number Of Dots Equals.
To Facilitate Our Understanding Of How Valence Electrons Interact, A Simple Way Of Representing Those Valence Electrons Would Be Useful.
Web Lewis Structures (Also Known As Lewis Dot Structures Or Electron Dot Structures) Are Diagrams That Represent The Valence Electrons Of Atoms Within A Molecule.
Web A Video Tutorial For How To Draw Lewis Structures In Five Steps.
Related Post:
.PNG)