Gmp Organization Chart
Gmp Organization Chart - Fo r the manufacture of medication, drug pro ducts, several. Which may broadly be categorized into two groups: Web gmp professional or great training material for the newbie. Web gmp good manufacturing practices gqa global quality audit gvp good pharmacovigilance practices gxp combined term for gcp, gdp, gclp, glp, gmp,. Click this image to get pdf file of organization chart. Web a substance, other than the active pharmaceutical ingredient (api), which has been appropriately evaluated for safety and is included in a medicines delivery system to: A committee of the participating. Web download scientific diagram | standard organizational chart for a gmp production site from publication: The organization chart for the. Web the processing parameters for all steps must be sufficiently detailed to permit complete reproducibility of the process each time it is performed: Web gmp good manufacturing practices gqa global quality audit gvp good pharmacovigilance practices gxp combined term for gcp, gdp, gclp, glp, gmp,. Web they use process control charts to monitor the process and procedures in order to manage variability. As the pic scheme is an arrangement between regulatory authorities, it is very flexible, dynamic and proactive. The organization chart for. The 10 golden rules of gmp pharmout pty ltd, abn: Which may broadly be categorized into two groups: Web it is important to define the job responsibilities of all the personnel as it an important part of gmp. Gmp organization chart and the document describing the quality assurance system 4. Web the processing parameters for all steps must be sufficiently. 85 117 673 766, unit 10, 24 lakeside. Click this image to get pdf file of organization chart. Web senior management should establish a quality policy that describes the overall intentions and direction of the company related to quality and should ensure continuing suitability. Web good manufacturing practices (gmp, also referred to as 'cgmp' or 'current good manufacturing practice') is. Environment and occupational safety and quality. Web organizational chart showing the arrangements for quality assurance, including production and quality control qualification, experience, and responsibilities of key personnel. Web gmp is a collection of g uidelines, codes, and regulations. Web good manufacturing practice (gmp) is the part of quality assurance (qa) that : (as of july 1, 2023) Web gmp is aimed primarily at diminishing the risks inherent in any pharmaceutical production; Web senior management should establish a quality policy that describes the overall intentions and direction of the company related to quality and should ensure continuing suitability. Click this image to get pdf file of organization chart. Web they use process control charts to monitor the process. (as of july 1, 2023) Web gmp is a collection of g uidelines, codes, and regulations. Web good manufacturing practices (gmp, also referred to as 'cgmp' or 'current good manufacturing practice') is the aspect of quality assurance that ensures that medicinal. Fo r the manufacture of medication, drug pro ducts, several. The organization chart for the. Web gmp good manufacturing practices gqa global quality audit gvp good pharmacovigilance practices gxp combined term for gcp, gdp, gclp, glp, gmp,. Web good manufacturing practices (gmp, also referred to as 'cgmp' or 'current good manufacturing practice') is the aspect of quality assurance that ensures that medicinal. Web download scientific diagram | standard organizational chart for a gmp production site. Web gmp is aimed primarily at diminishing the risks inherent in any pharmaceutical production; Fo r the manufacture of medication, drug pro ducts, several. Web good manufacturing practices or gmp is a system that consists of processes, procedures and documentation that ensures manufacturing products, such as food, cosmetics, and. The 10 golden rules of gmp pharmout pty ltd, abn: Manufacturing. Fo r the manufacture of medication, drug pro ducts, several. Manufacturing flow chart and detailed description of the actual. Web gmp is a collection of g uidelines, codes, and regulations. Web good manufacturing practices or gmp is a system that consists of processes, procedures and documentation that ensures manufacturing products, such as food, cosmetics, and. Web a substance, other than. Web organizational chart showing the arrangements for quality assurance, including production and quality control qualification, experience, and responsibilities of key personnel. Web download scientific diagram | standard organizational chart for a gmp production site from publication: Environment and occupational safety and quality. Web gmp good manufacturing practices gqa global quality audit gvp good pharmacovigilance practices gxp combined term for gcp,. Environment and occupational safety and quality. Gmp organization chart and the document describing the quality assurance system 4. Click this image to get pdf file of organization chart. Web good manufacturing practice (gmp) is the part of quality assurance (qa) that : Web download scientific diagram | standard organizational chart for a gmp production site from publication: Web senior management should establish a quality policy that describes the overall intentions and direction of the company related to quality and should ensure continuing suitability. As the pic scheme is an arrangement between regulatory authorities, it is very flexible, dynamic and proactive. Web organizational chart showing the arrangements for quality assurance, including production and quality control qualification, experience, and responsibilities of key personnel. Web in this on demand course (part 2 of 11), you will learn the elements for ensuring organizational structure and workforce, as required by regulation. (as of july 1, 2023) Web a substance, other than the active pharmaceutical ingredient (api), which has been appropriately evaluated for safety and is included in a medicines delivery system to: Job responsibilities should contain all the works the person is. Web gmp professional or great training material for the newbie. Web the processing parameters for all steps must be sufficiently detailed to permit complete reproducibility of the process each time it is performed: A committee of the participating. 85 117 673 766, unit 10, 24 lakeside.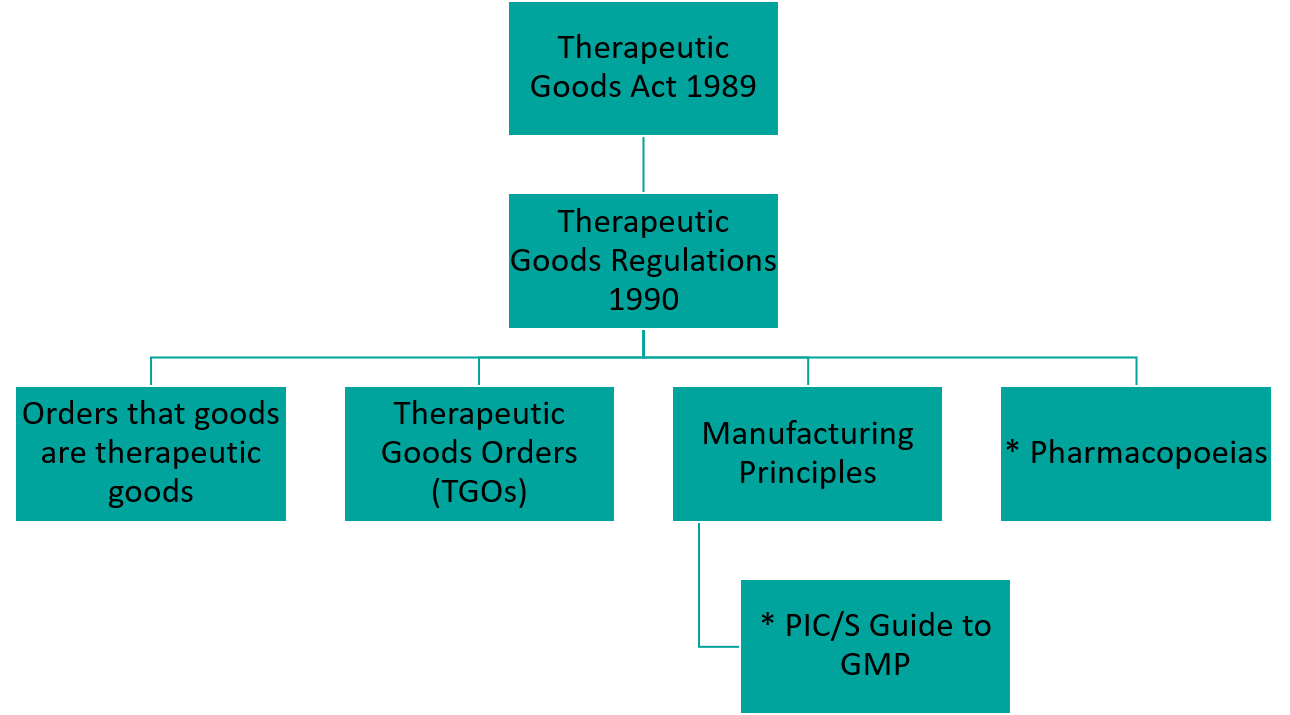
Navigating the Pathway of TGA Enforcement of the PIC/S Guide to GMP

GMP Organization Chart

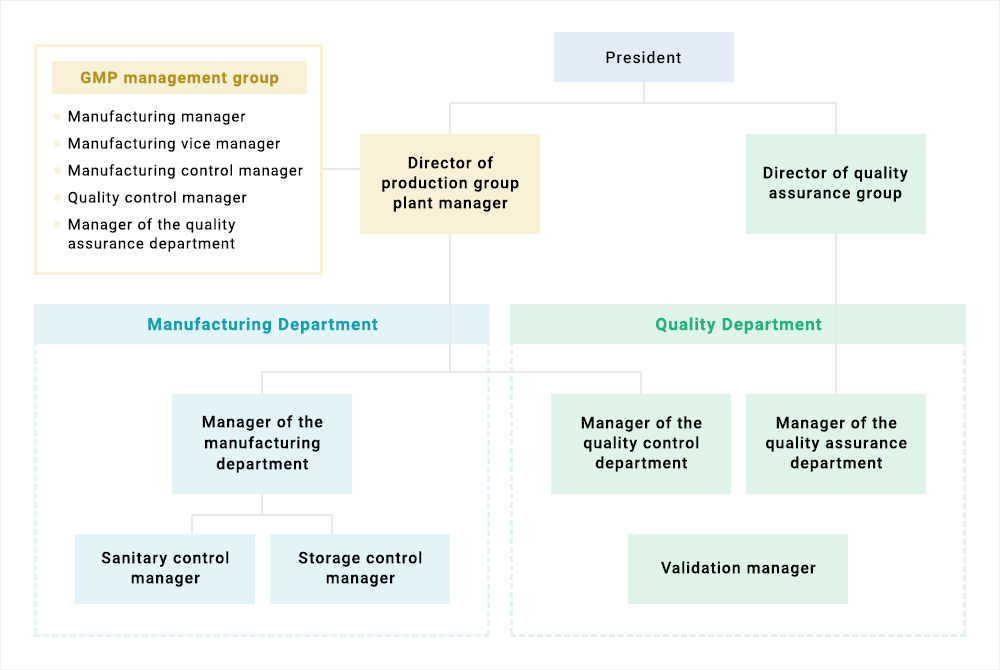
Standard organizational chart for a GMP production site Download
Comply with GMP conditions for manufacturing safe and effective APIs

A Review on Good Manufacturing Practice (GMP) for Medicinal Products
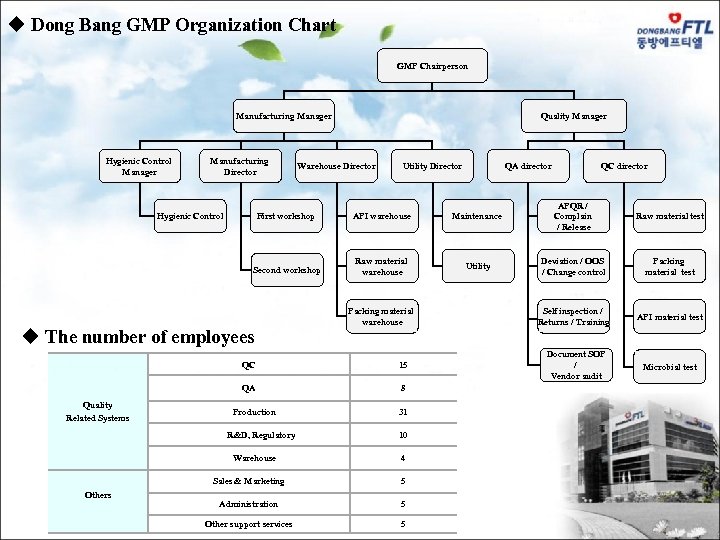
GMP Organization Chart
R&D Reliability TSUKIOKA FILM PHARMA CO., LTD.
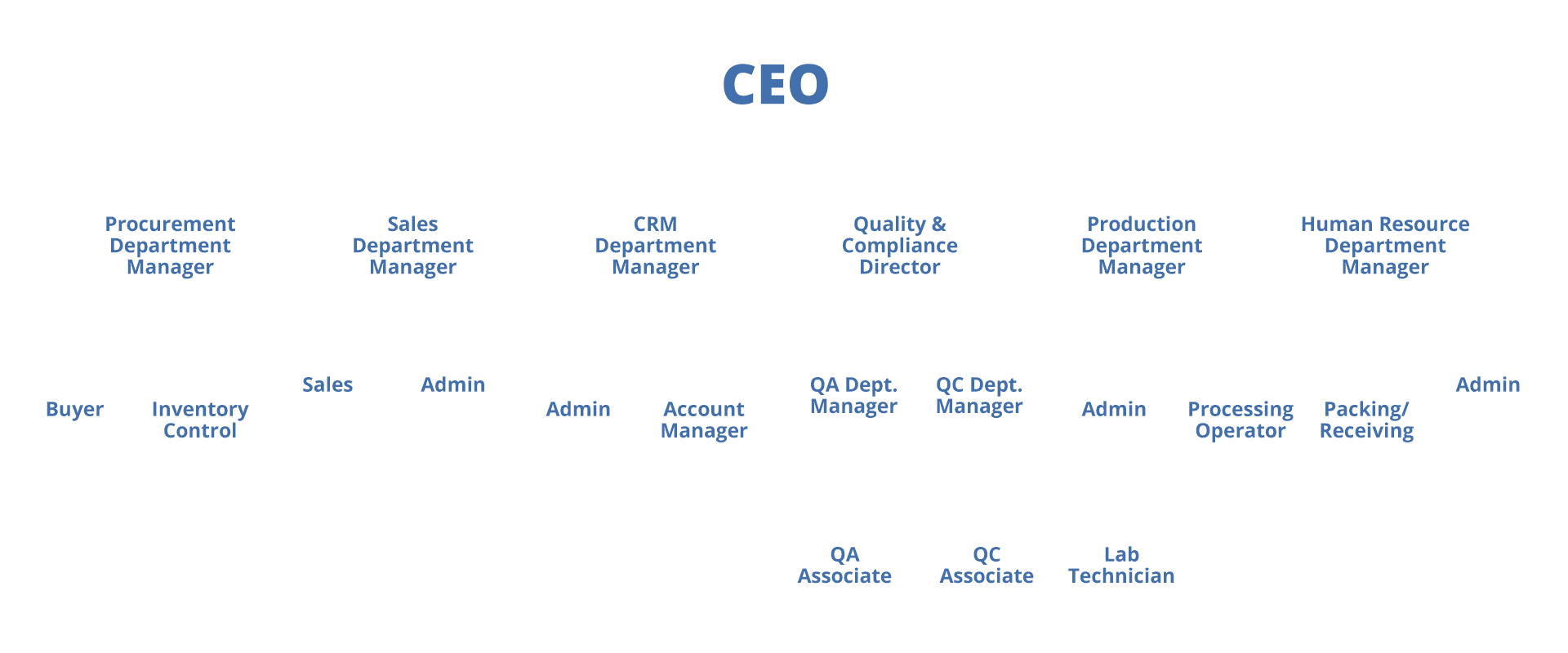
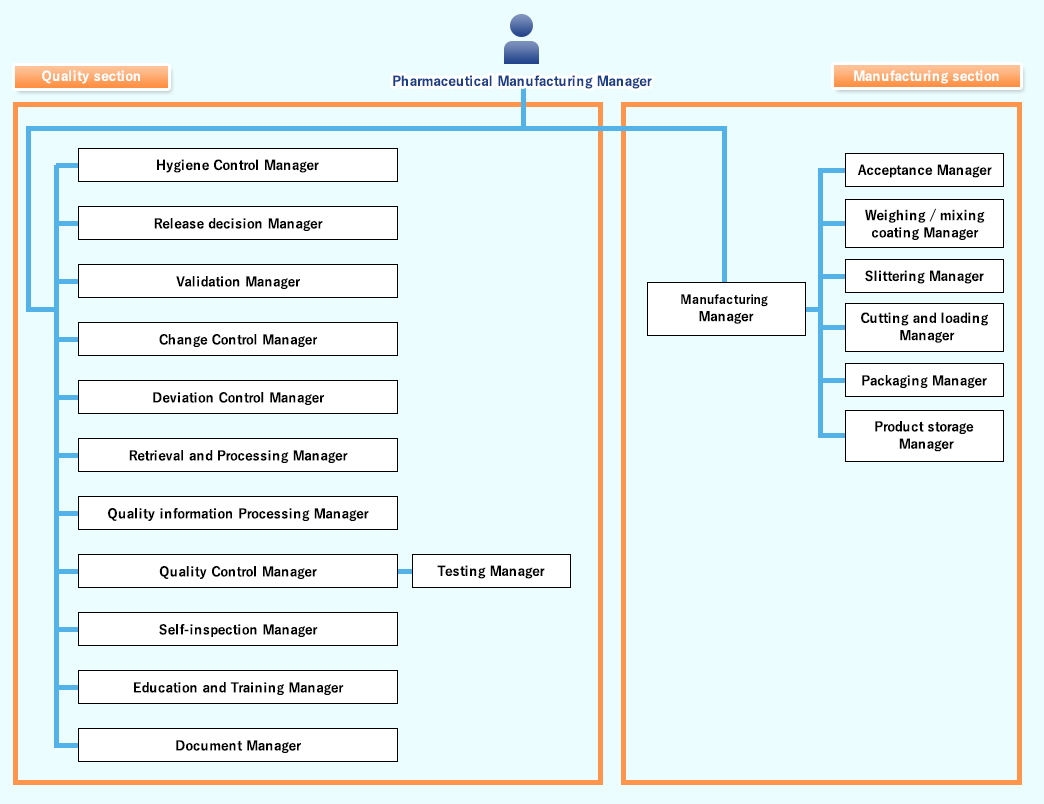
GMP Organization Chart
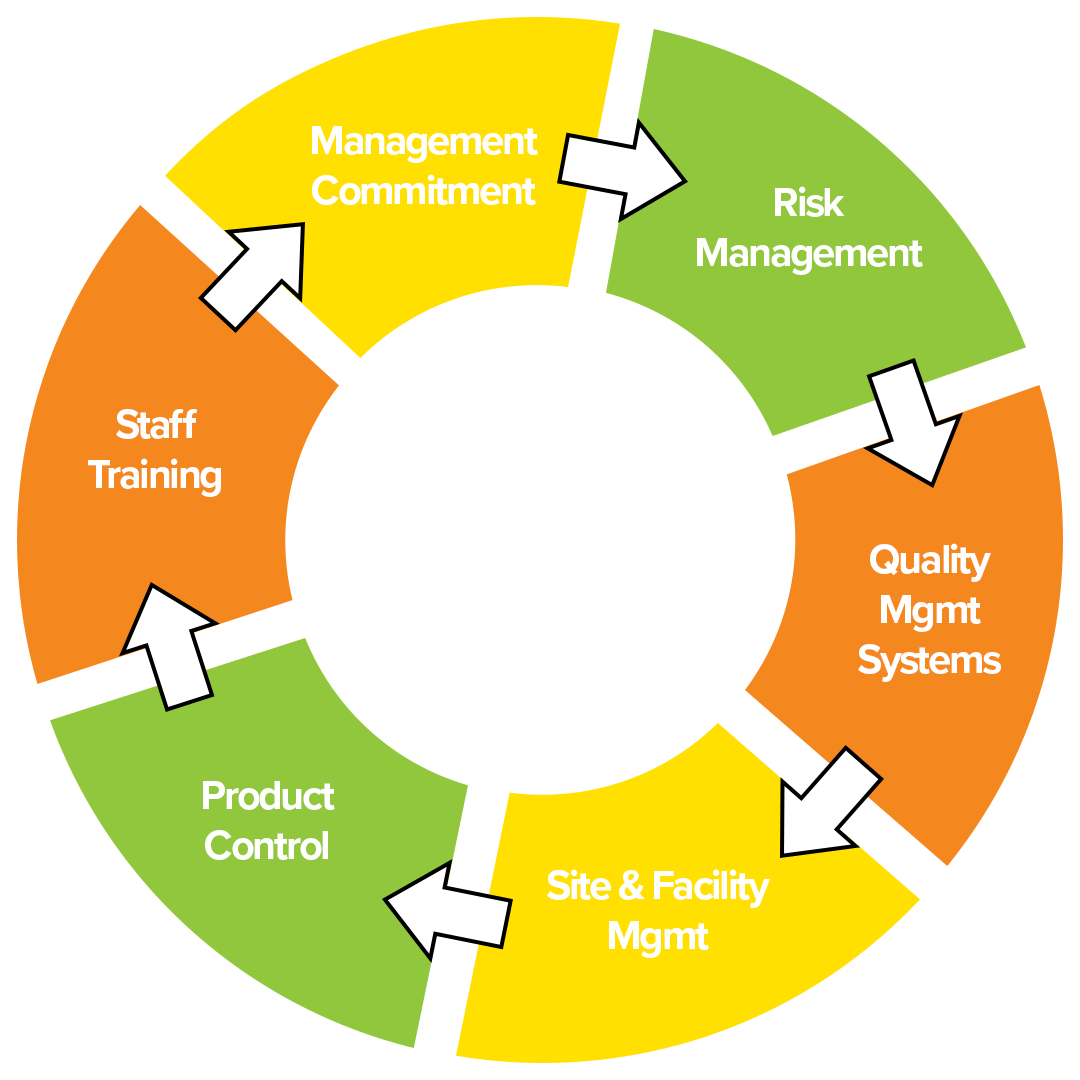
Good Manufacturing Practice (GMP) Resources Mr Techhno
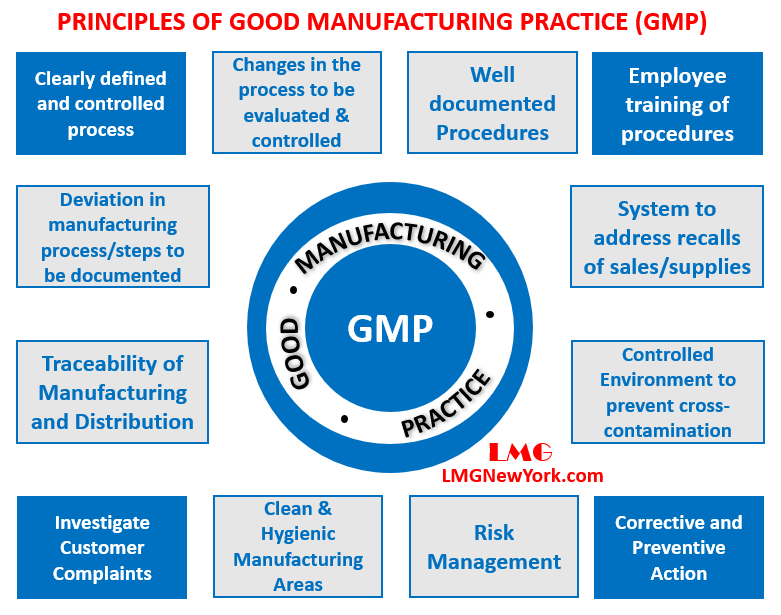
GMP Good Manufacturing Practice LMG New York
Web Gmp Is Aimed Primarily At Diminishing The Risks Inherent In Any Pharmaceutical Production;
The 10 Golden Rules Of Gmp Pharmout Pty Ltd, Abn:
The Purpose Of The Quality Systems Is To Ensure Quality.
Web Gmp Good Manufacturing Practices Gqa Global Quality Audit Gvp Good Pharmacovigilance Practices Gxp Combined Term For Gcp, Gdp, Gclp, Glp, Gmp,.
Related Post: