Gas Laws Chart
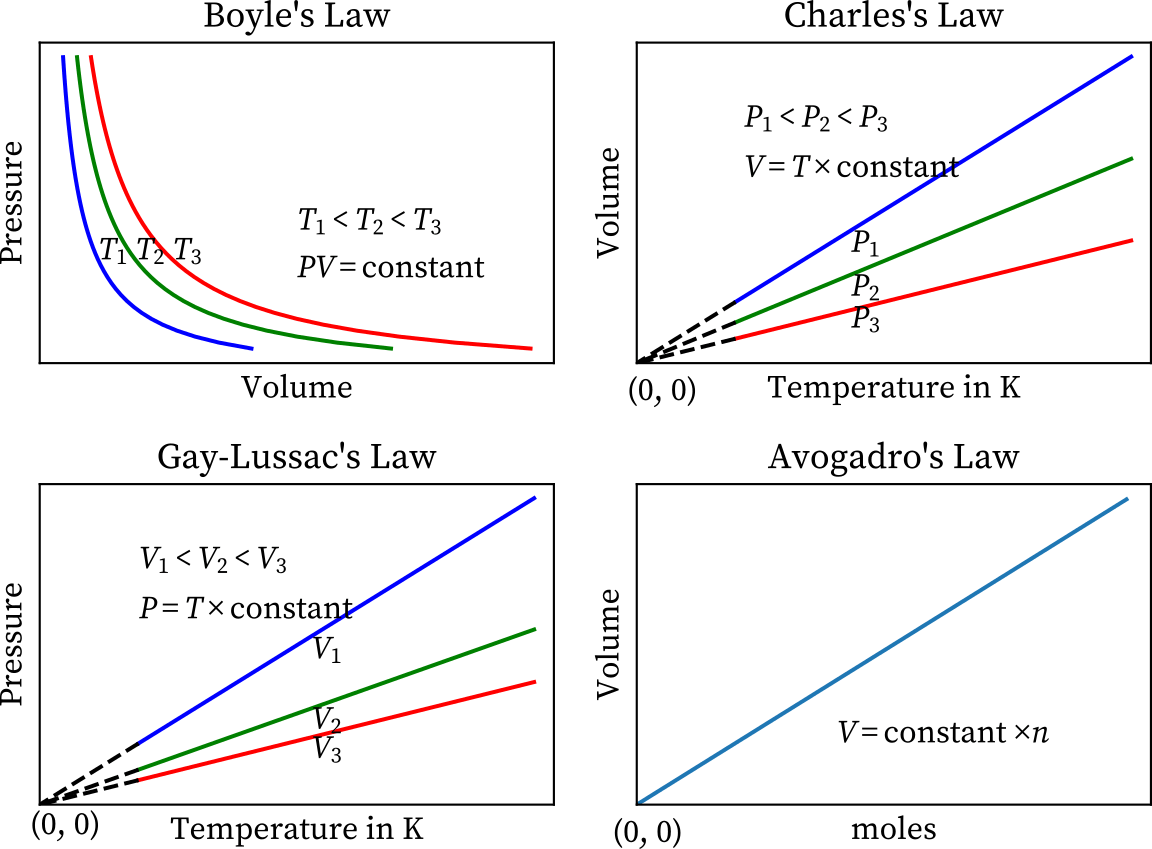
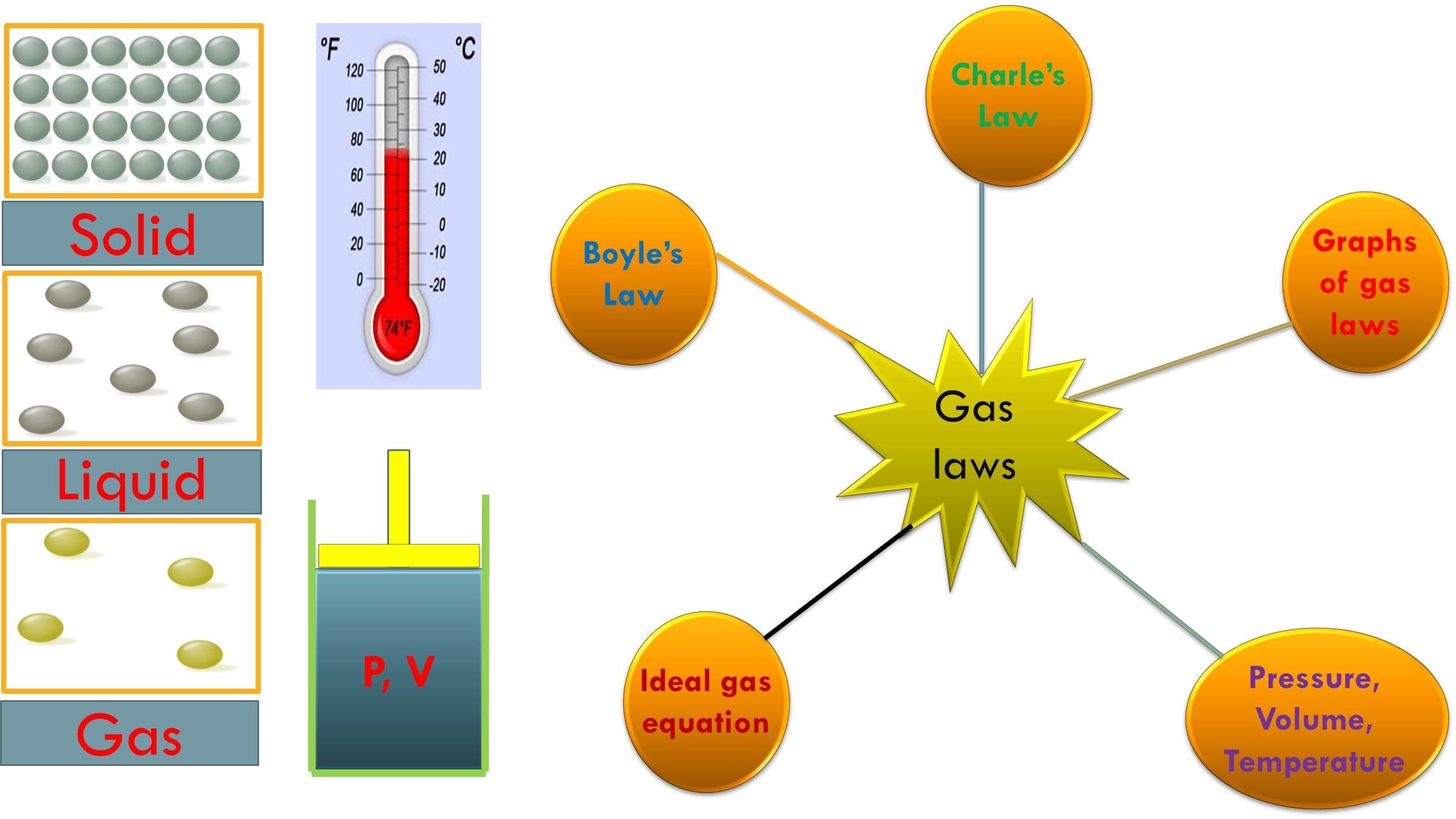
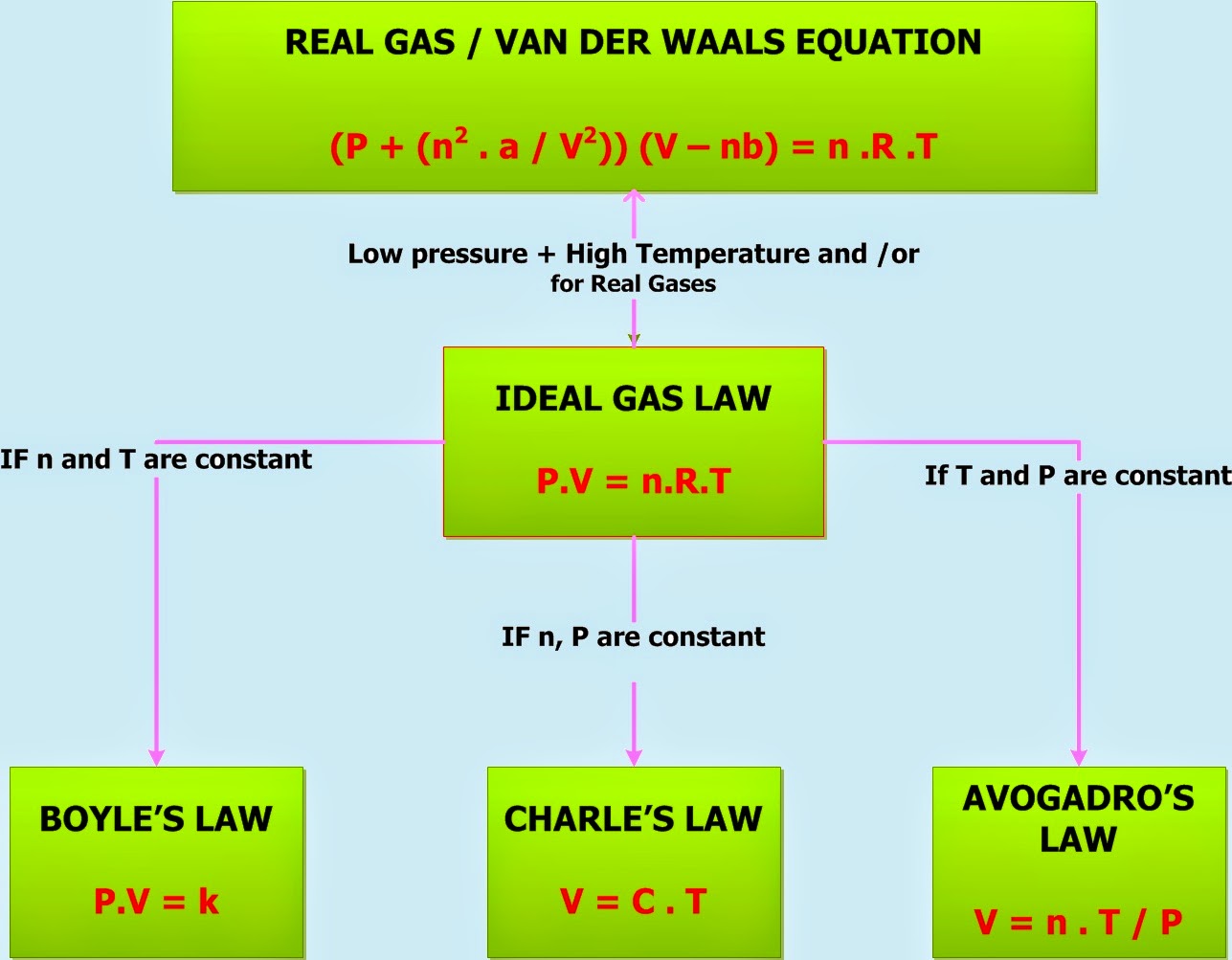
Gas Laws Chart - Principle that warm air is less dense than cooler air. While it is important to understand the relationships covered by each law, knowing the originator is not as important and will be rendered redundant once the combined gas law is introduced. Web p1v1 = p2v2 = constant. Web there are 4 general laws that relate the 4 basic characteristic properties of gases to each other. · try some practice worksheets. Web the ideal gas law is often written in an empirical form: Boyle's law relates a gas's pressure and volume at constant temperature and amount. Web the five gas laws are listed below: Charles's law relates a gas's volume and temperature at constant pressure and amount. The gas laws in chemistry are: Web the gas laws consist of three primary laws: Standard temperature and pressure (stp) are a useful set of benchmark conditions to compare other properties of gases. Typically, these problems aren't so much about doing the math once you. The ideal gas law can be used in stoichiometry problems whose chemical reactions involve gases. Boyle's law, charles' law, the combined. The partial pressure of vapor molecules above the surface of a liquid at equilibrium at a given temperature is the vapor pressure (vp) of the liquid at that temperature. Unit conversions for the gas laws. Web the gas laws consist of three primary laws: Standard temperature and pressure (stp) are a useful set of benchmark conditions to compare other properties. Lots of students have trouble when doing gas law calculations. Web the gas laws are empirical laws that describe the properties of gases and typically include avogadro's law, boyle's law, and charles' laws and dalton's laws. Typically, these problems aren't so much about doing the math once you. Standard temperature and pressure (stp) are a useful set of benchmark conditions. It provides a relationship between the pressure and the volume of a gas. These laws show how a change in one of these properties affects the others. The ideal gas law can be used in stoichiometry problems whose chemical reactions involve gases. Robert boyle conducted an experiment on gases to study the deviation of its behaviour in changed physical conditions.. Web a logical corollary to avogadro's hypothesis (sometimes called avogadro’s law) describes the relationship between the volume and the amount of a gas: The ideal gas law can be used in stoichiometry problems whose chemical reactions involve gases. Web as the temperature is raised, the fraction of molecules that can break away into the gas phase grows and vapor pressure. A hot air balloon works on the. Web • in chemistry, the relationships between gas physical properties are described as gas laws. Charles' law, boyle's law and avogadro's law (all of which will later combine into the general gas equation and ideal gas law). At stp, gases have a volume of 22.4 l per. Each law is titled by its. Complete the following tables, showing your work for each lettered box beside the corresponding letter below. Boyle’s law states the relation between volume and pressure at constant temperature and mass. Unit conversions for the gas laws. Web a logical corollary to avogadro's hypothesis (sometimes called avogadro’s law) describes the relationship between the volume and the amount of a gas: The. Web the ideal gas law is the equation of state of a hypothetical ideal gas. Web the mathematical modeling of relationships between variables such as the number of gas particles, the temperature of a gas, the volume of the gas container, gas constants, and the pressure of the gas, helps students to progressively expand and derive the gas laws from. Each law is titled by its discoverer. Web as the temperature is raised, the fraction of molecules that can break away into the gas phase grows and vapor pressure increases. Boyle’s law describes the inverse relationship between the pressure and volume of a fixed amount of gas at a constant temperature. Web a logical corollary to avogadro's hypothesis (sometimes called. While it is important to understand the relationships covered by each law, knowing the originator is not as important and will be rendered redundant once the combined gas law is introduced. · try some practice worksheets. Boyle’s law states the relation between volume and pressure at constant temperature and mass. Web p1v1 = p2v2 = constant. According to it, the. Web a logical corollary to avogadro's hypothesis (sometimes called avogadro’s law) describes the relationship between the volume and the amount of a gas: Web the ideal gas law is often written in an empirical form: Suppose p is the pressure and v is the volume, then. Unit conversions for the gas laws. Web there are 4 general laws that relate the 4 basic characteristic properties of gases to each other. These laws show how a change in one of these properties affects the others. Web the ideal gas law is the equation of state of a hypothetical ideal gas. However, a number of rates available to. Web the mathematical modeling of relationships between variables such as the number of gas particles, the temperature of a gas, the volume of the gas container, gas constants, and the pressure of the gas, helps students to progressively expand and derive the gas laws from experimental data. It provides a relationship between the volume occupied by a gas and the absolute temperature. Typically, these problems aren't so much about doing the math once you. It states that under a constant temperature when the pressure on a gas increases its volume decreases. How do you fill up a hot air balloon? The gas laws in chemistry are: And is the ideal gas constant. Web as the temperature is raised, the fraction of molecules that can break away into the gas phase grows and vapor pressure increases.
Combined Gas Law — Overview & Calculations Expii

Pin by Joni Loomis on Chemistry (With images) Ideal gas law, Gas laws

Gas Laws Environmental Chemistry

OCR A2 Physics Gas Laws Andrew PoverAndrew Pover
Universal Gas Law Study Guide Inspirit Learning Inc
The Gas Laws Definition, Formula & Examples StudiousGuy
Gas Laws Ideal Gas Law Chemistry Net

The Theories and Behavior of Gas Owlcation

Charle’s vs Boyle’s Gas Laws anchor chart Chemistry classroom
Gas Laws CK12 Foundation
Web Coverage Of Empirical Gas Laws And The Two State Approach To Solving Problems Involving A Change In State Variables.
Web The Gas Laws Consist Of Three Primary Laws:
Web The Five Gas Laws Are Listed Below:
Charles' Law, Boyle's Law And Avogadro's Law (All Of Which Will Later Combine Into The General Gas Equation And Ideal Gas Law).
Related Post:
