Galvanic Action Chart
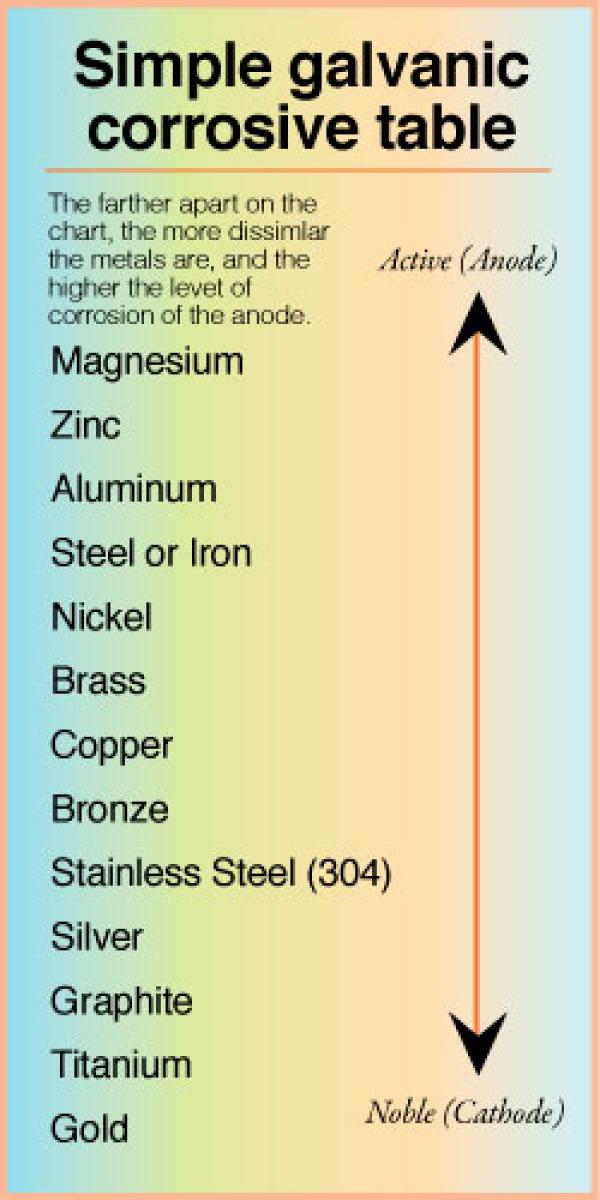
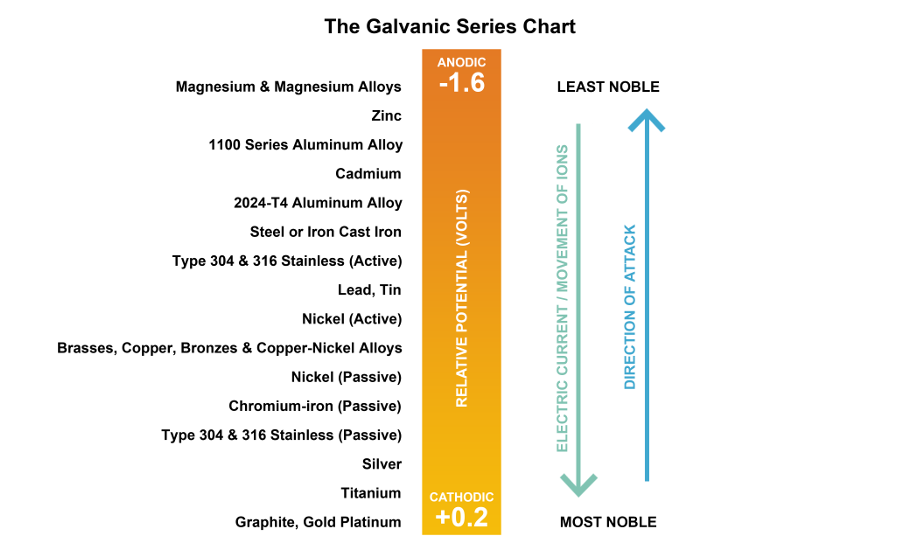
Galvanic Action Chart - It is not always true that there is greater corrosion the further down the scale one goes. Note that there are several tables presented on this page and that they do not all agree. When dissimilar metals are used together in the presence of an electrolyte, separate them with a dielectric material such as insulation, paint or similar surface coating. Web when dissimilar metals are connected — either by simple contact or by wiring — and they are immersed in water, a current will flow which can cause galvanic corrosion. In certain cases one metal immediately following another may be very corrosive. When is stainless steel passive or active. Web galvanic corrosion happens when two conductive metals (anode and cathode) are in contact and exposed to an electrolyte with a return current path. Web a chart depicting the galvanic series for some common metals in a frequently encountered conducting solution, seawater, is included in figure 1. Web galvanic corrosion is a localised mechanism by which metals can be preferentially corroded. We also provide other helpful methods for avoiding galvanic corrosion. Web schematic diagram of galvanic corrosion principle. Web the galvanic action increases as the metals are farther apart in the galvanic series. When dissimilar metals are used together in the presence of an electrolyte, separate them with a dielectric material such as insulation, paint or similar surface coating. To minimize galvanic corrosion, select fasteners based on their material compatibility with. The closer together the material are on the chart to the right, the less galvanic action will. Web by simply choosing metals that avoid galvanic action there will never be an issue. Most architects know enough about it to be dangerous, but what exactly causes this breakdown? Web galvanic corrosion (also called bimetallic corrosion or dissimilar metal corrosion) is an. Web below is a galvanic reaction chart for dissimilar metals. This form of corrosion has the potential to attack junctions of metals, or regions where one construction metal contacts another. There are three conditions that must exist for galvanic corrosion to occur. The list begins with the more active (anodic) metal and proceeds down. Web read on to find out. Web fastened of galvanic corrosion in joint, it’s recommended to choose materials that are grouped together in the galvanic series chart. Web below is a galvanic reaction chart for dissimilar metals. Below is a galvanic reaction chart for dissimilar metals. The below galvanic corrion chart or anodic index table shows anodic index for different materials. Galvanic voltages relative to gold. This chart is designed to assist in broadly assessing the risk of galvanic corrosion associated with a given metal coming into contact with another metal. Simpletwig will then try to. Web galvanic corrosion happens when two conductive metals (anode and cathode) are in contact and exposed to an electrolyte with a return current path. Below is a galvanic reaction chart. Web a chart depicting the galvanic series for some common metals in a frequently encountered conducting solution, seawater, is included in figure 1. First there must be two electrochemically dissimilar metals present. Web galvanic corrosion happens when two conductive metals (anode and cathode) are in contact and exposed to an electrolyte with a return current path. Web galvanic corrosion (also. This chart is designed to assist in broadly assessing the risk of galvanic corrosion associated with a given metal coming into contact with another metal. Tabular representation of the galvanic series. Web when dissimilar metals are connected — either by simple contact or by wiring — and they are immersed in water, a current will flow which can cause galvanic. Select materials that are as close together as possible in the galvanic series chart. Below is a galvanic reaction chart for dissimilar metals. What is the galvanic series? We also provide other helpful methods for avoiding galvanic corrosion. The list begins with the more active (anodic) metal and proceeds down. It is not always true that there is greater corrosion the further down the scale one goes. Web a chart depicting the galvanic series for some common metals in a frequently encountered conducting solution, seawater, is included in figure 1. This chart is designed to assist in broadly assessing the risk of galvanic corrosion associated with a given metal coming. Tabular representation of the galvanic series. This form of corrosion has the potential to attack junctions of metals, or regions where one construction metal contacts another. What is the galvanic series? Types of galvanic corrosion in different metals and their alloys. Web galvanic corrosion (some times called dissimilar metal corrosion) is the process by which the materials in contact with. What is the galvanic series? The below galvanic corrion chart or anodic index table shows anodic index for different materials. Web schematic diagram of galvanic corrosion principle. What are the causes of galvanic corrosion? When dissimilar metals are used together in the presence of an electrolyte, separate them with a dielectric material such as insulation, paint or similar surface coating. Most architects know enough about it to be dangerous, but what exactly causes this breakdown? Though the order of metals in a galvanic series remains the same in most conducting solutions, some. This chart is designed to assist in broadly assessing the risk of galvanic corrosion associated with a given metal coming into contact with another metal. In this article, we'll look at an example to illustrate the use of the galvanic table. Galvanic voltages relative to gold. Simpletwig will then try to. Web what is galvanic corrosion? When is stainless steel passive or active. In this respect, one should understand how to read the following chart which lists all metals. If you're a designer working with exterior metals, you've probably heard of galvanic corrosion. The closer together the material are on the chart to the right, the less galvanic action will.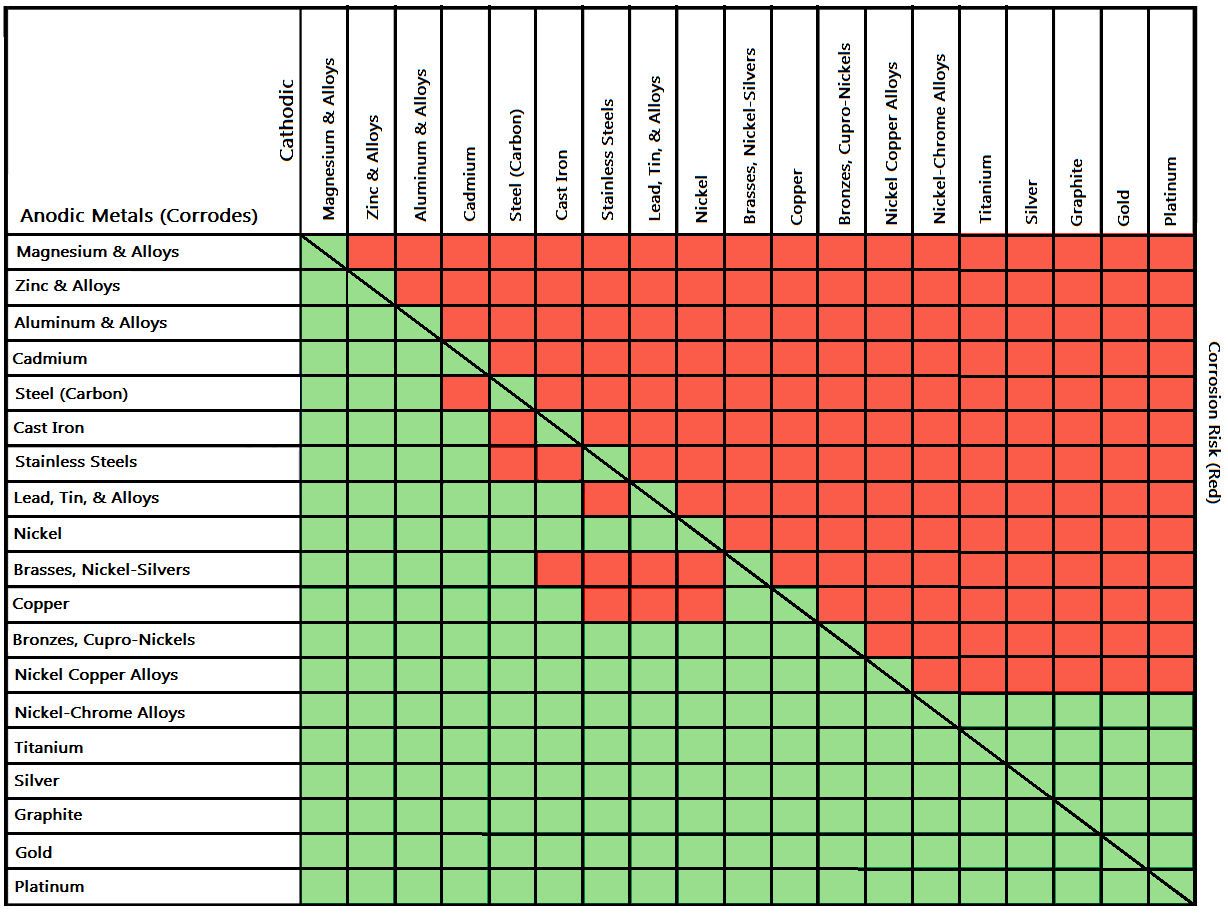
Galvanic Corrosion Common Questions Answered
Galvanic Corrosion Chart Metals

Prevent Galvanic Action Frame Building News

Galvanic Reaction Chart All Points Fasteners
Galvanic Action Chart PDF
Galvanic Chart Of Metals

Galvanic Corrosion Chart Dissimilar Metals Video Bokep Ngentot
Galvanic Corrosion Compatibility Chart
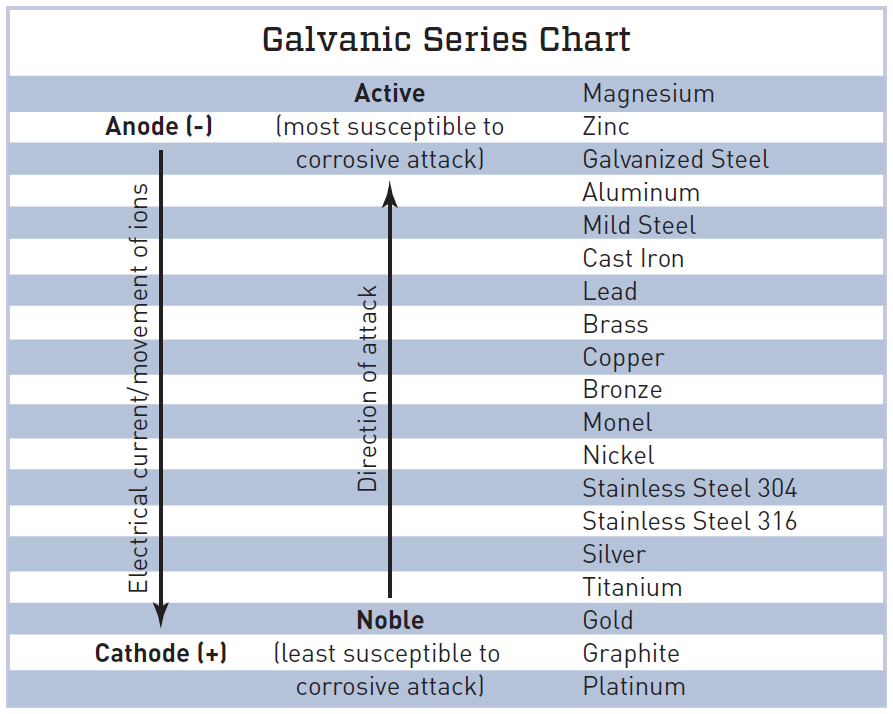
Galvanic Series Chart

Galvanic Action Corrosion Prevention Architect's Blog
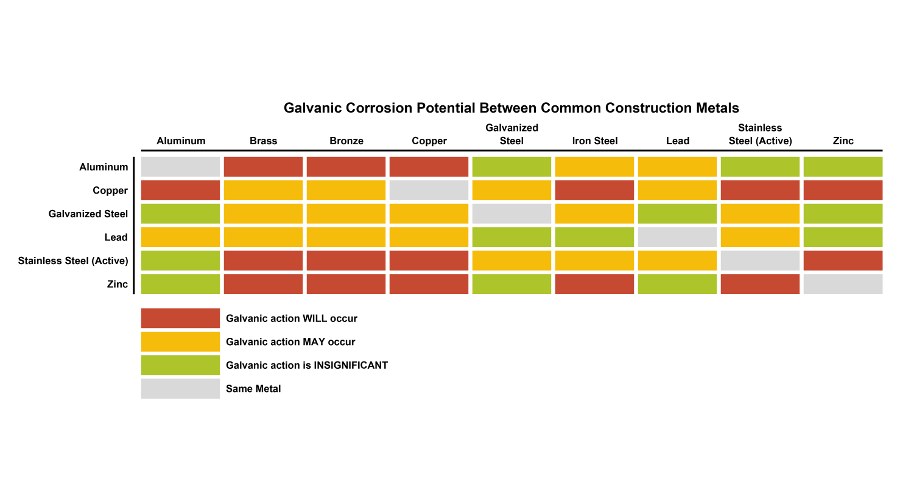
Web This Slide Includes A Chart Of Galvanic Corrosion Potential Between Common Construction Metals.
Web Galvanic Corrosion Happens When Two Conductive Metals (Anode And Cathode) Are In Contact And Exposed To An Electrolyte With A Return Current Path.
Web When Dissimilar Metals Are Connected — Either By Simple Contact Or By Wiring — And They Are Immersed In Water, A Current Will Flow Which Can Cause Galvanic Corrosion.
The Metallic Phases, Compounds, Dilution Or Enrichment Regions Of Component Elements, And Oxidation Films In The Alloy Showing Different Electrode Potentials May Also Be Galvanically With The Metal, And Deactivation And Concentration Effects Can Also Form A Galvanic.
Related Post:
