Exelon Patch Placement Chart
Exelon Patch Placement Chart - 4.6 mg/24 hours 9.5 mg/24 hours : In that case, return it to your pharmacist. 1 indications and usage 1.1 alzheimer’s disease exelon patchis indicated for the treatment of dementia of the alzheimer’s type(ad).efficacy has been demonstrated in patients with mild, moderate,andsevere alzheimer’s disease. Apply a new patch if it falls off. If the patch falls off, put a new one on. Web the outside of the backing layer is beige and labelled for each patch as follows: Only 1 exelon patch should be worn at a time. Each transdermal patch of 5 cm2 contains 9 mg rivastigmine. Do not use exelon patch if the packaging is torn or shows signs of tampering. Treatment is started with exelon® patch 5 applied once a day. 1 indications and usage 1.1 alzheimer’s disease exelon patchis indicated for the treatment of dementia of the alzheimer’s type(ad).efficacy has been demonstrated in patients with mild, moderate,andsevere alzheimer’s disease. Each exelon patch is sealed in a pouch that protects it until you are ready to put it on (see figure a). Treatment with exelon patch was initiated at 4.6 mg/24. Each exelon patch is sealed in a pouch that protects it until you are ready to put it on (see figure a). 9 mg 18 mg ; One transdermal patch is sealed in one sachet. Rivastigmine transdermal patch each patch of 10 cm2contains 18 mg rivastigmine base, in vivo release rate of 9.5 mg/24 h. Apply a new patch if. Web exelon® (rivastigmine hydrogen tartrate) page 1 of 62 product monograph prexelon® patch 5 rivastigmine transdermal patch each patch of 5 cm2 contains 9 mg rivastigmine base, in vivo release rate of 4.6 mg/24 h. Web the outside of the backing layer is beige and labelled for each patch as follows: One transdermal patch is sealed in one sachet. Web. Each transdermal patch of 5 cm2 contains 9 mg rivastigmine. Replace with a new patch every 24 hours. Apply a new patch if it falls off. Prexelon® patch 10 rivastigmine transdermal patch each patch of 10 cm2 contains 18 mg rivastigmine. Each 5 cm2 patch contains 9 mg rivastigmine base, with in vivo release rate of 4.6 mg/24 hours. 4.6 mg/24 hours 9.5 mg/24 hours : Web exelon patch is a prescription medicine used to treat: Prexelon® patch 10 rivastigmine transdermal patch each patch of 10 cm2 contains 18 mg rivastigmine. Web exelon® (rivastigmine hydrogen tartrate) page 1 of 62 product monograph prexelon® patch 5 rivastigmine transdermal patch each patch of 5 cm2 contains 9 mg rivastigmine base, in. So, based on the above formula, the roe for exelon is: Web move the patch site with each new patch. After a minimum of 4 weeks of treatment and if well tolerated, this dose should be increased to exelon patch. Below is a guide to rotating of sites but it does not replace your responsibility in ensuing you have all. In that case, return it to your pharmacist. Return on equity = net profit (from continuing operations) ÷ shareholders' equity. Web exelon patch is a prescription medicine used to treat: Web exelon patch is supplied in cartons containing 30 patches (see figure a) figure a. Below is a guide to rotating of sites but it does not replace your responsibility. 9 mg 18 mg ; 4.6 mg/24 hours 9.5 mg/24 hours : Replace with a new patch every 24 hours. Table 1 summarizes the available strengths and quantity of rivastigmine provided in each patch: • if you have any questions or require more information, please read the package information leaflet that came with the medicine. Wearing more than 1 patch at a time can lead to very bad and sometimes deadly overdose. Front right upper arm front left upper arm right abdomen left abdomen back right upper arm back left upper arm front right upper arm front left upper arm right side upper arm left side upper arm right front chest left. If you use. The patch should stay in place, even when you are showering, bathing, or swimming. Web exelon ® patch (rivastigmine transdermal system) 4.6 mg/24 hours, 9.5 mg/24 hours, and 13.3 mg/24 hours is indicated for the treatment of mild to moderate dementia of the alzheimer's type and mild to moderate dementia associated with parkinson's disease. If the patch falls off, put. Return on equity = net profit (from continuing operations) ÷ shareholders' equity. Web press the patch firmly in place for 30 seconds with the palm of your hand to make sure that the edges of the patch stick well. Replace with a new patch every 24 hours. Only 1 exelon patch should be worn at a time. Below is a guide to rotating of sites but it does not replace your responsibility in ensuing you have all the Web exelon ® patch (rivastigmine transdermal system) 4.6 mg/24 hours, 9.5 mg/24 hours, and 13.3 mg/24 hours is indicated for the treatment of mild to moderate dementia of the alzheimer's type and mild to moderate dementia associated with parkinson's disease. Do not use exelon patch in children. Each exelon patch is sealed in a pouch that protects it until you are ready to put it on (see figure a). Web move the patch site with each new patch. Of these, 256 have been treated for at least 12 weeks, 232 for at least 24 weeks, and 196 for at least 52 weeks. Put patch on at the same time of day. Web exelon patch can only be placed on certain areas of the body including the legs, arms,. In that case, return it to your pharmacist. Web exelon patch is a prescription medicine used to treat: So, based on the above formula, the roe for exelon is: 4.6 mg/24 hours 9.5 mg/24 hours :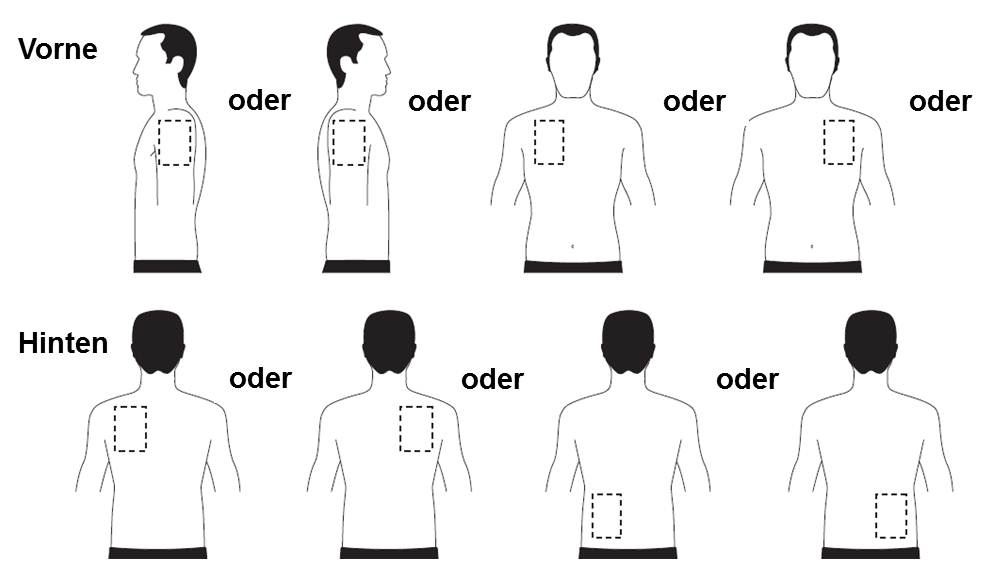
Exelon Patch 5 Matrixpfl 4.6 Mg/24h 30 Stück in der Adler Apotheke
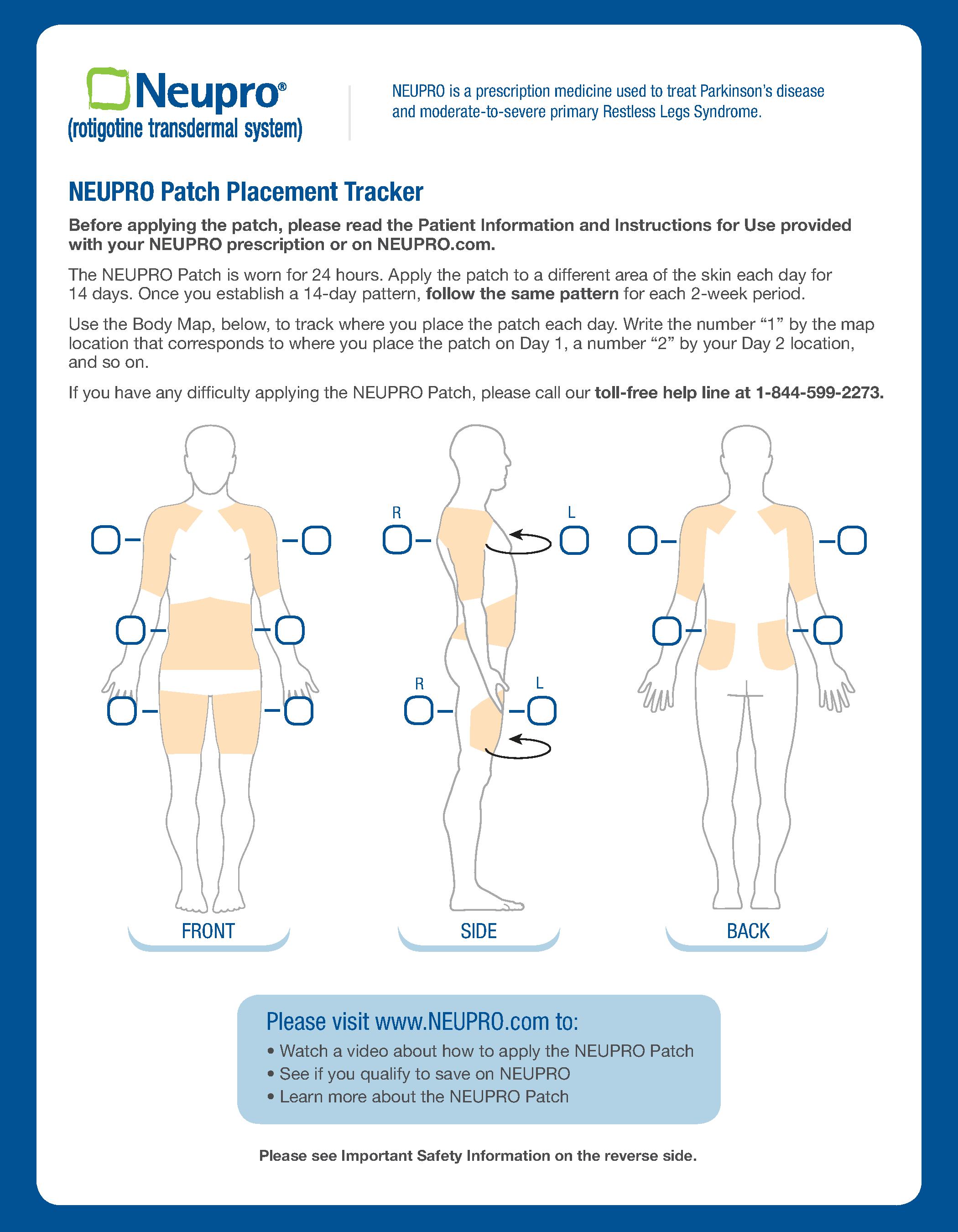
Patch Placement Tracker (1/2) — NEUPRO
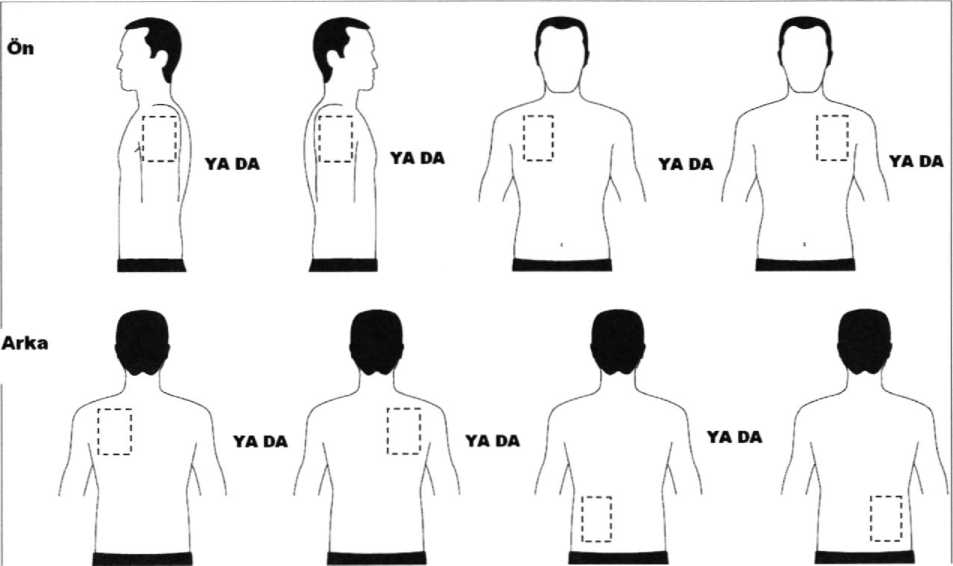
Exelon Patch 5 Transdermal Flaster Kullanma Talimatı İlaç Prospektüsü

EMAR Cheat Sheet The Grand Healthcare System
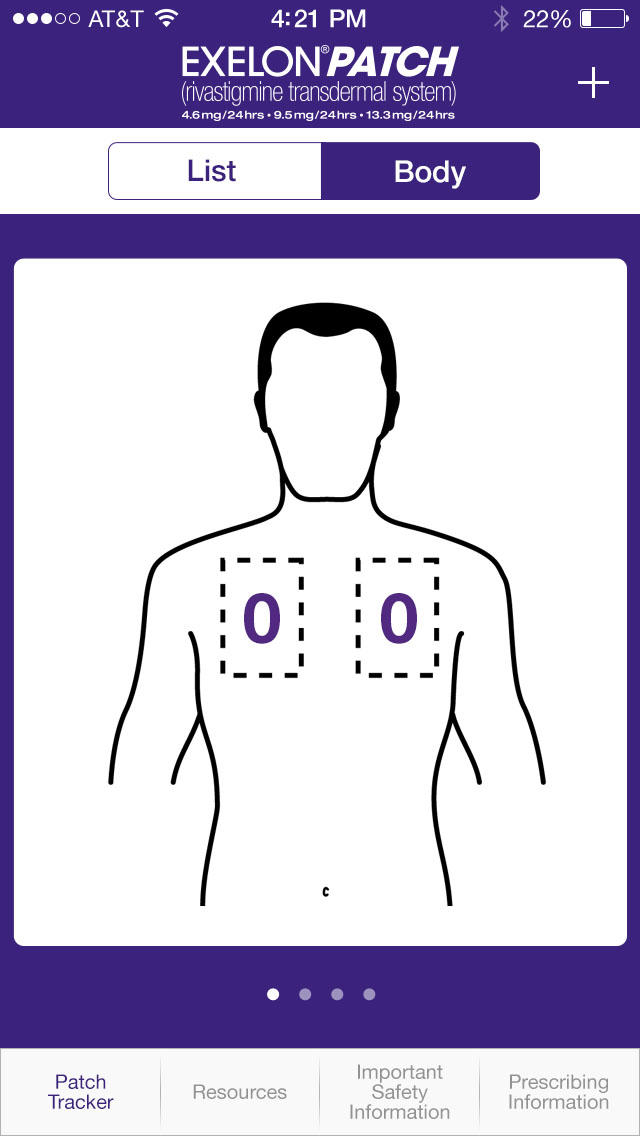
POCKET.MD PatchMate, Keep track of your loved one’s EXELON PATCH

New Exelon®Patch Now Available in 2 Strengths from Novartis

POCKET.MD PatchMate, Keep track of your loved one’s EXELON PATCH
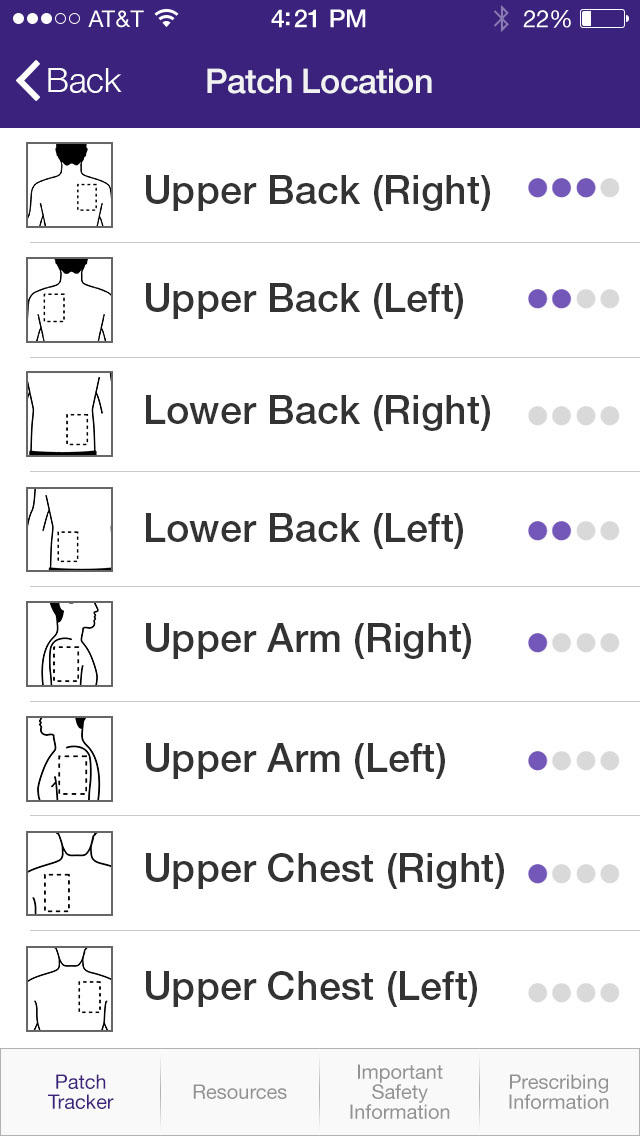
POCKET.MD PatchMate, Keep track of your loved one’s EXELON PATCH

Exelon Patch FDA prescribing information, side effects and uses
Exelon Patch Placement Chart
Rivastigmine Transdermal Patch Each Patch Of 5 Cm2 Contains 9 Mg Rivastigmine Base, In Vivo Release Rate Of 4.6 Mg/24 H.
After A Minimum Of 4 Weeks Of Treatment And If Well Tolerated, This Dose Should Be Increased To Exelon Patch.
If You Use It After The Expiry Date Has Passed, It May Not Work As Well As It Should.
Do Not Use Exelon Patch If The Packaging Is Torn Or Shows Signs Of Tampering.
Related Post: