Drawing Hybridized Orbitals
Drawing Hybridized Orbitals - Sp³ hybridized orbitals and sigma bonds. Sp² hybridized orbitals and pi bonds. Hybrid orbitals overlap to form σ bonds. This type of hybridization is required whenever an atom is surrounded by three groups of electrons. Recall the valence electron configuration of a carbon atom: Want to join the conversation? The carbon atoms of c2h2 are sp hybridized. In sp hybridization, one s orbital and one p orbital hybridize to form two sp orbitals, each consisting of 50% s character and 50% p character. Describe the hybrid orbitals used in the formation of bonding for each atom in some carbon containing compounds. The three sp2 hybrids are arranged with trigonal planar geometry, pointing to the three corners of an equilateral triangle, with angles of 120° between them. Sp3 hybrid orbitals and tetrahedral bonding. Organic chemistry > unit 1. Read through the provided information, and sketch the lewis dot diagram of the provided compound. Web drawing hybrid orbitals on central atom. The three sp2 hybrids are arranged with trigonal planar geometry, pointing to the three corners of an equilateral triangle, with angles of 120° between them. Make sure to get the hybridization correct! Web when we say that the two electrons from each of the hydrogen atoms are shared to form a covalent bond between the two atoms, what we mean in valence bond theory terms is that the two spherical 1 s orbitals overlap, allowing the two electrons to form a pair within the two. Organic chemistry > unit 1. Web drawing hybrid orbitals on central atom. Organic chemistry > unit 1. Draw orbital box diagrams showing how combinations of an atomic s orbital and various numbers of p orbitals create sp, sp 2, and sp 3 hybrid orbitals. All orbitals in a set of hybrid orbitals are equivalent in shape and energy. In sp³ hybridization, one s orbital and three p orbitals hybridize to form four sp³ orbitals, each consisting of 25% s character and 75% p character. This 109.5 o arrangement gives tetrahedral geometry (figure 4). In the following sections, we shall discuss the common types of hybrid orbitals. Hybrid orbitals overlap to form σ bonds. Draw orbital box diagrams showing. Unhybridized orbitals overlap to form π bonds. This type of hybridization is required whenever an atom is surrounded by two groups of electrons. 1.9m views 3 years ago new ap & general chemistry video playlist. Draw orbital box diagrams showing how combinations of an atomic s orbital and various numbers of p orbitals create sp, sp 2, and sp 3. Sp³ hybridized orbitals and sigma bonds. Now let’s look more carefully at bonding in organic molecules, starting with methane, ch 4. Want to join the conversation? This video explains how to decide what the hybridization around. Recall the valence electron configuration of a carbon atom: Now let’s look more carefully at bonding in organic molecules, starting with methane, ch 4. Pi bonding will occur when there is an. Sp² hybridized orbitals and pi bonds. Web in chemistry, orbital hybridisation (or hybridization) is the concept of mixing atomic orbitals to form new hybrid orbitals (with different energies, shapes, etc., than the component atomic orbitals) suitable for. Web when we say that the two electrons from each of the hydrogen atoms are shared to form a covalent bond between the two atoms, what we mean in valence bond theory terms is that the two spherical 1 s orbitals overlap, allowing the two electrons to form a pair within the two overlapping orbitals. Learn about sp² hybridized orbitals. This type of hybridization is required whenever an atom is surrounded by three groups of electrons. Organic chemistry > unit 1. Want to join the conversation? Sp³ hybridized orbitals and sigma bonds. 14k views 8 years ago general chemistry i. This type of hybridization is required whenever an atom is surrounded by four groups of electrons. This type of hybridization is required whenever an atom is surrounded by two groups of electrons. Hybrid orbitals overlap to form σ bonds. Learn about sp³ hybridized orbitals and sigma bonds. Web drawing hybrid orbitals on central atom. 14k views 8 years ago general chemistry i. In sp hybridization, one s orbital and one p orbital hybridize to form two sp orbitals, each consisting of 50% s character and 50% p character. 134k views 3 years ago. Hybrid orbitals overlap to form σ bonds. In sp² hybridization, one s orbital and two p orbitals hybridize to form three sp² orbitals, each consisting of 33% s character and 67% p character. Want to join the conversation? Want to join the conversation? Organic chemistry > unit 1. Learn about sp² hybridized orbitals and pi bonds. Web a set of hybrid orbitals is generated by combining atomic orbitals. Make sure to get the hybridization correct! Web in chemistry, orbital hybridisation (or hybridization) is the concept of mixing atomic orbitals to form new hybrid orbitals (with different energies, shapes, etc., than the component atomic orbitals) suitable for the pairing of electrons to form chemical bonds in valence bond theory. Web when we say that the two electrons from each of the hydrogen atoms are shared to form a covalent bond between the two atoms, what we mean in valence bond theory terms is that the two spherical 1 s orbitals overlap, allowing the two electrons to form a pair within the two overlapping orbitals. Web drawing hybrid orbitals on central atom. Sp3 hybrid orbitals and tetrahedral bonding. Learn about sp³ hybridized orbitals and sigma bonds.
Hybridization of Atomic Orbitals Sigma & Pi Bonds Sp Sp2 Sp3 YouTube
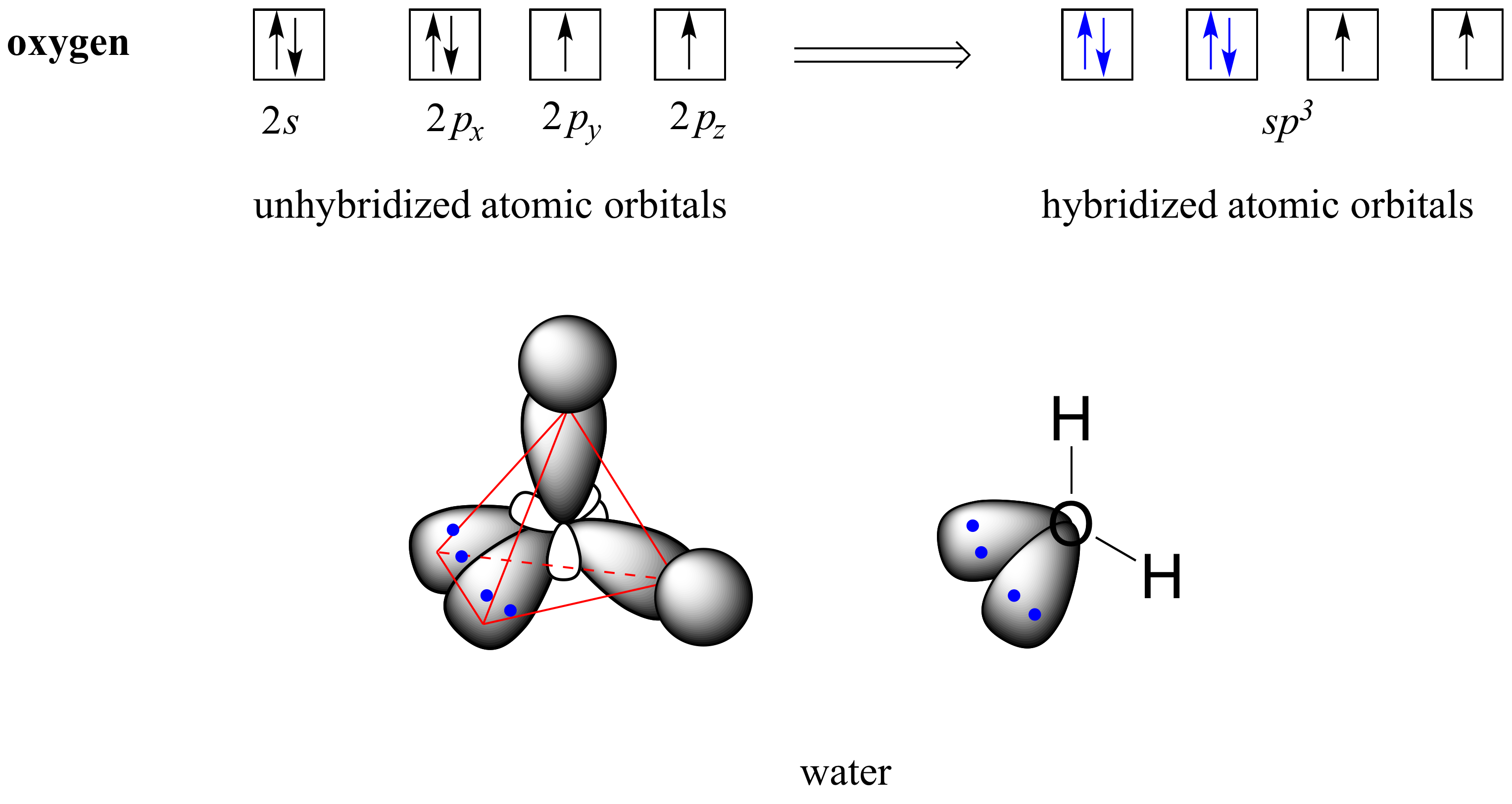
2.2 Hybrid orbitals Chemistry LibreTexts
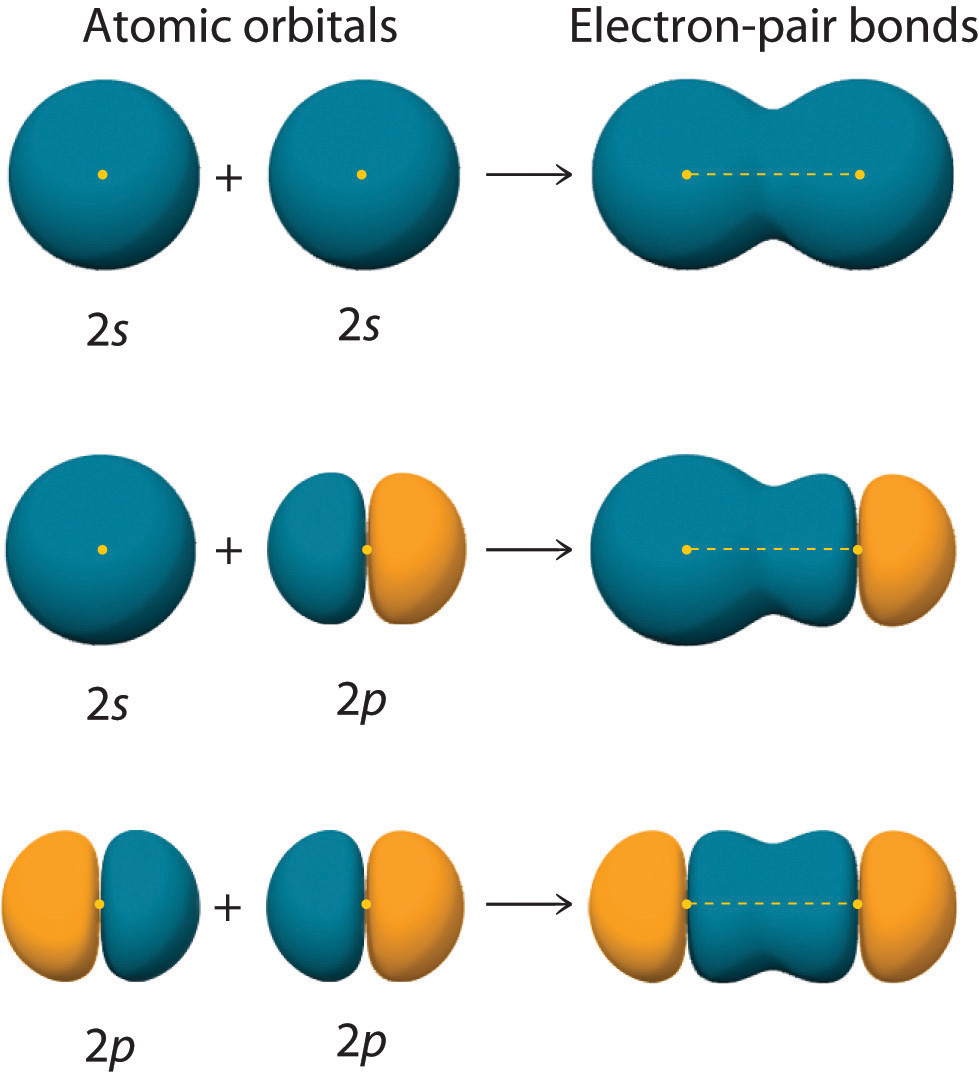
Chapter 6.2 Hybrid Orbitals Chemistry LibreTexts
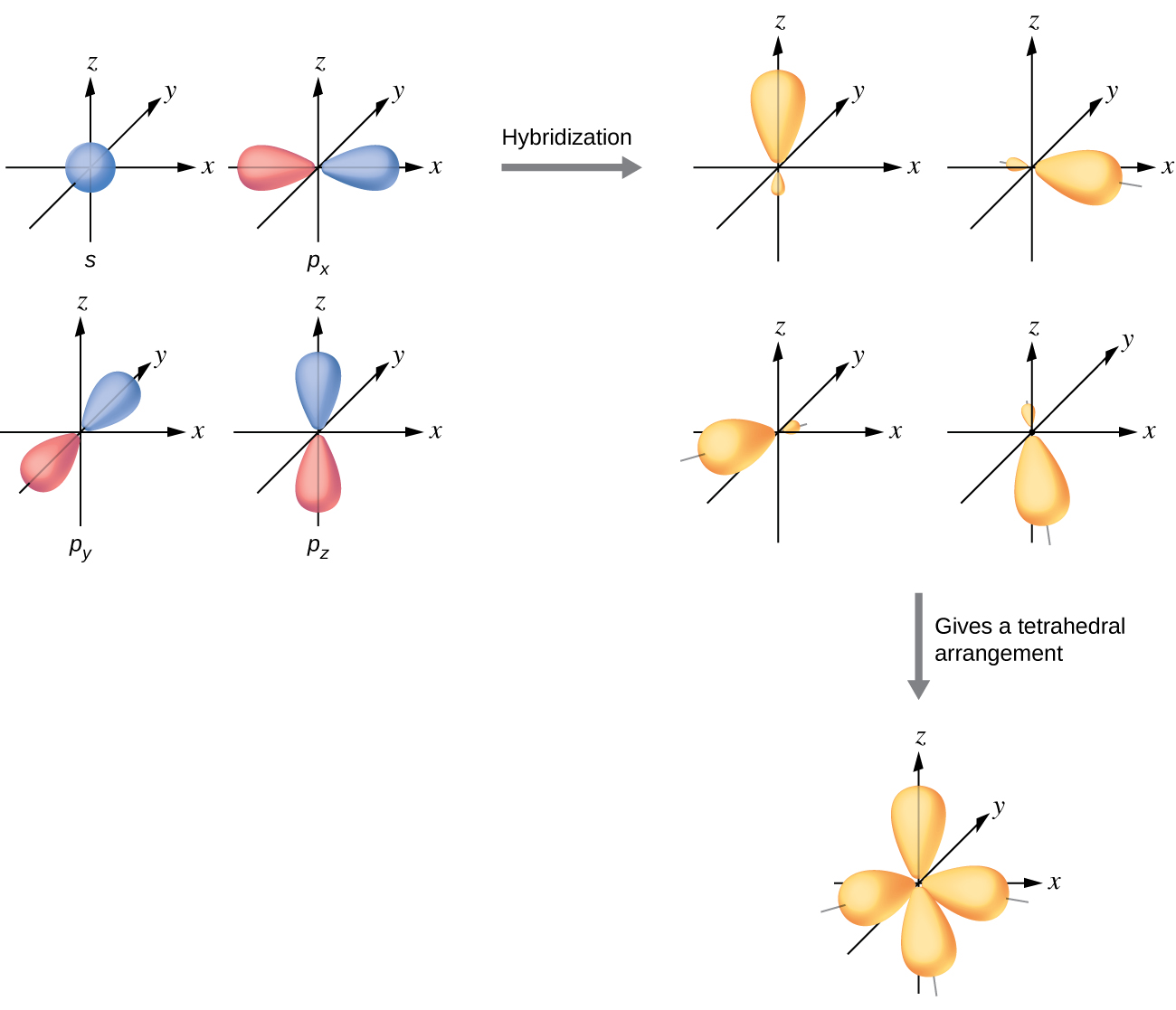
8.2 Hybrid Atomic Orbitals Chemistry
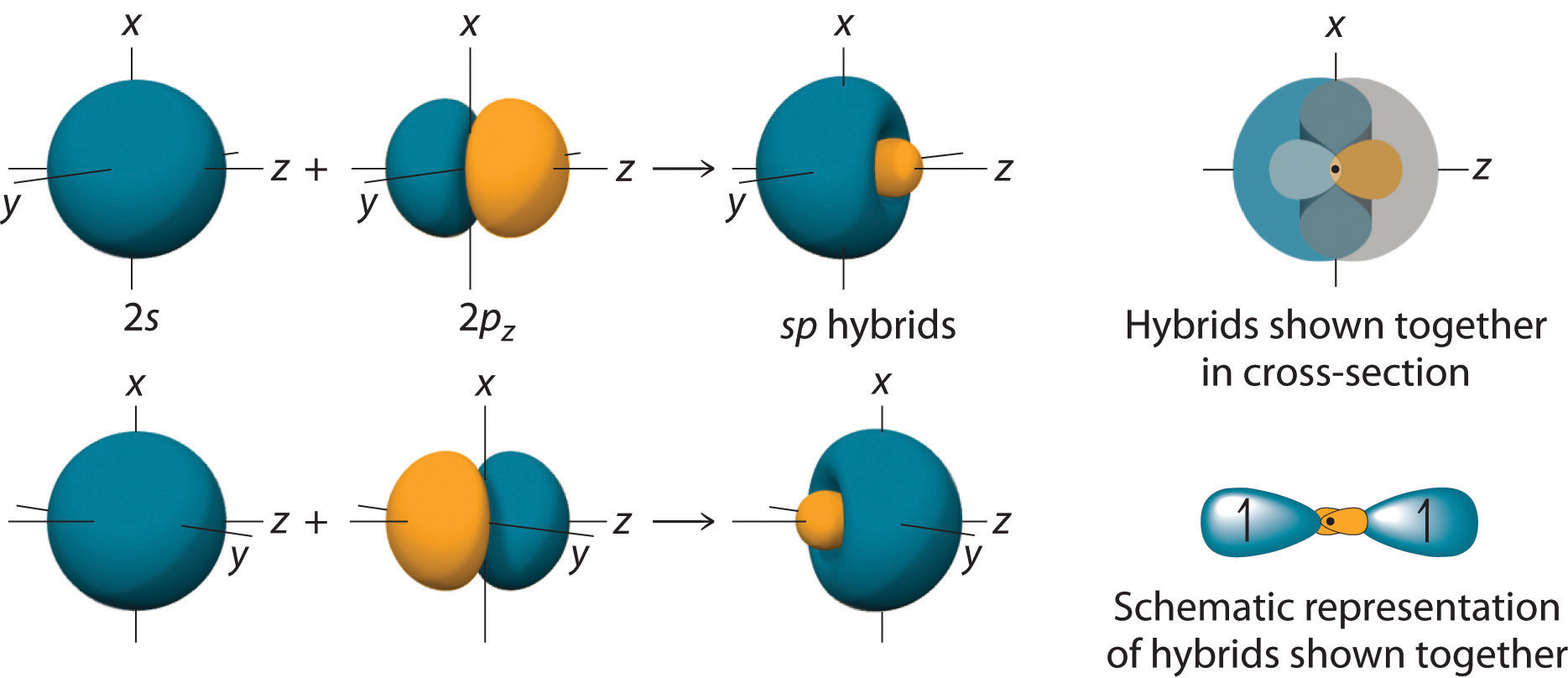
Localized Bonding and Hybrid Atomic Orbitals
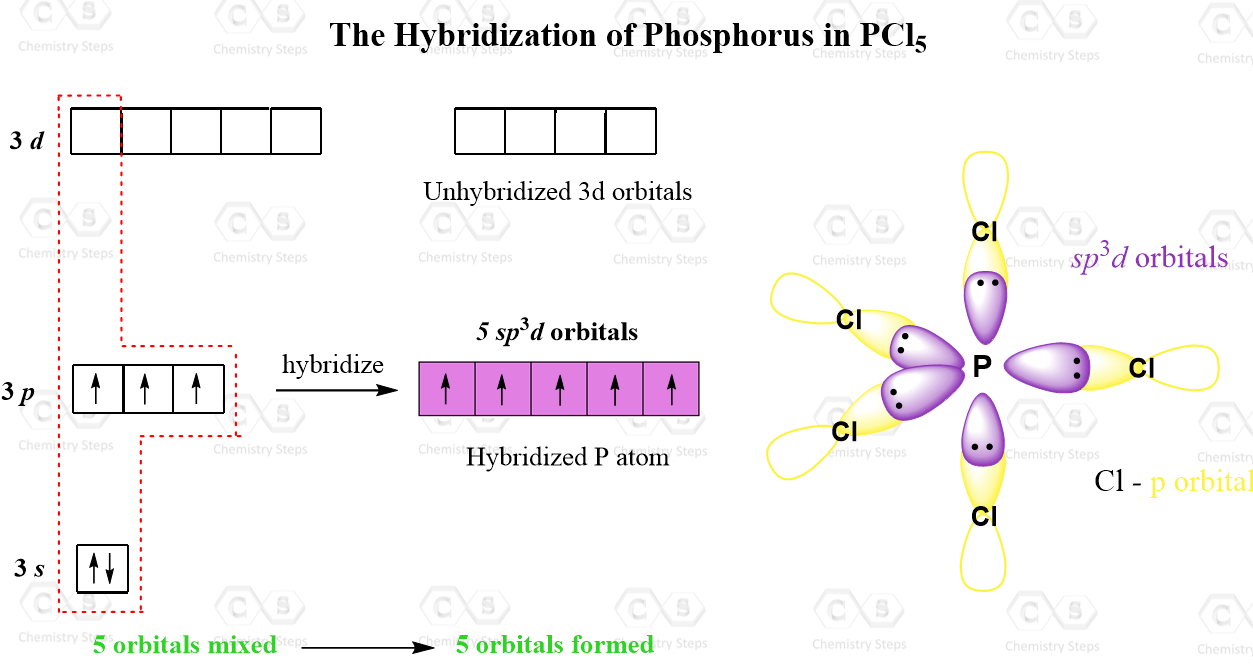
Hybridization of Atomic Orbitals Chemistry Steps
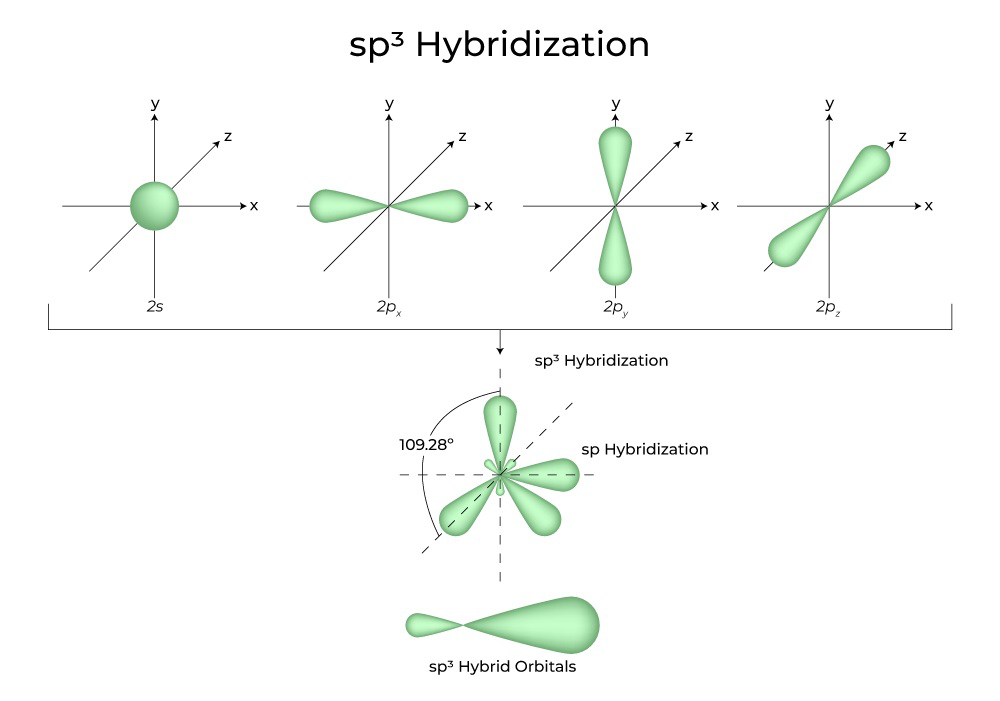
Sp Orbitals
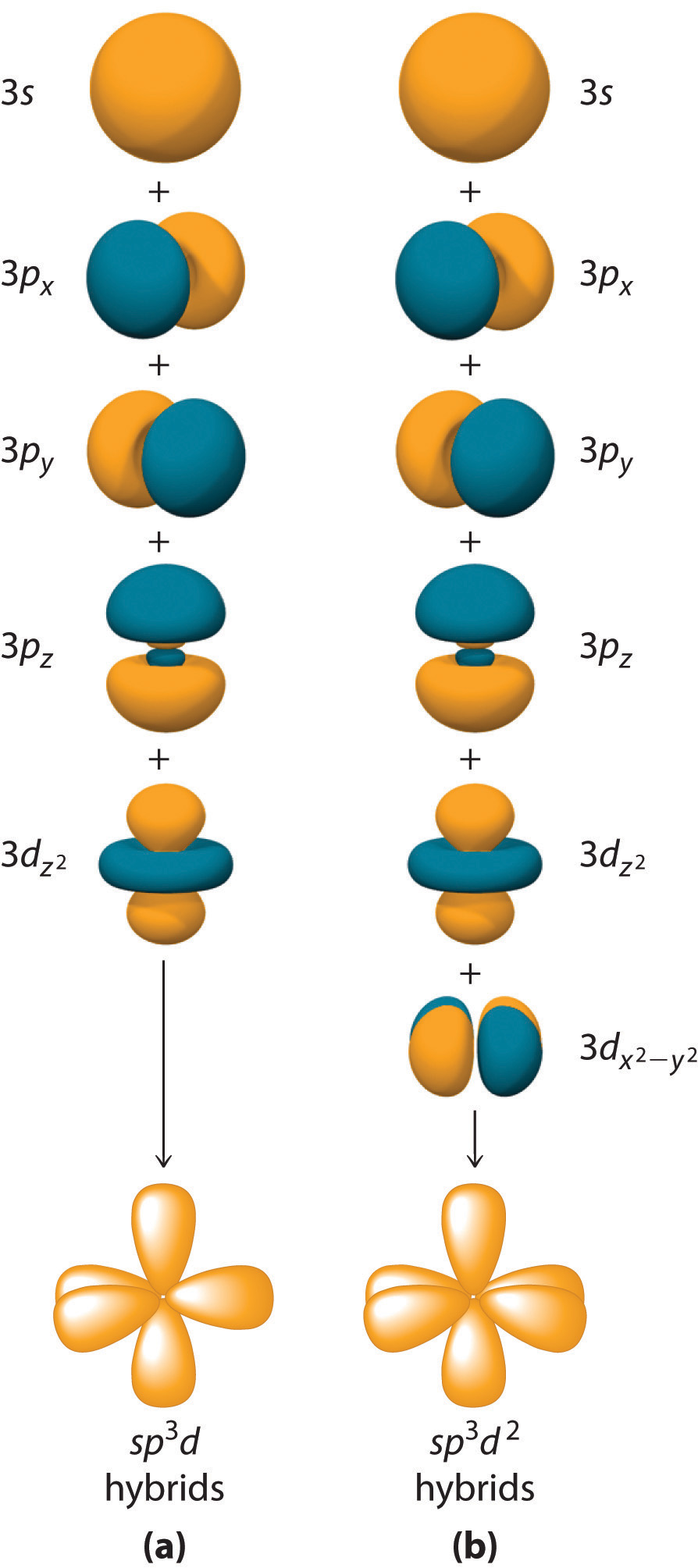
Localized Bonding and Hybrid Atomic Orbitals
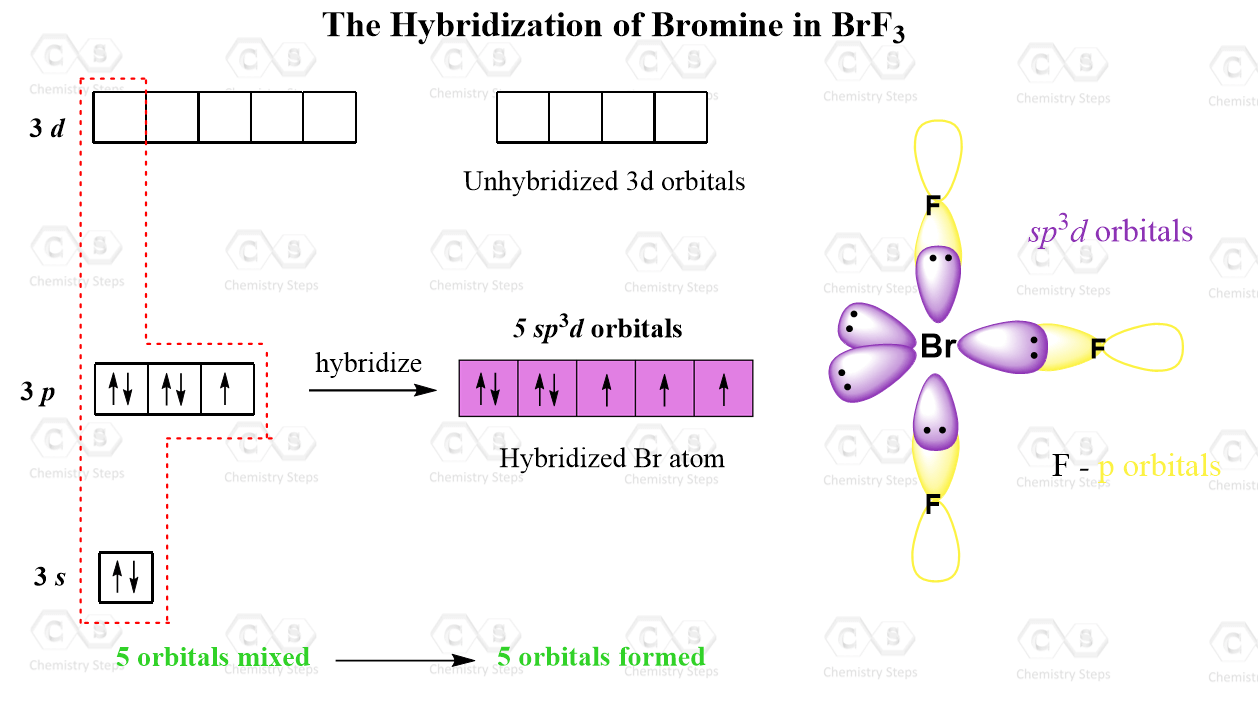
Hybridization of Atomic Orbitals Chemistry Steps
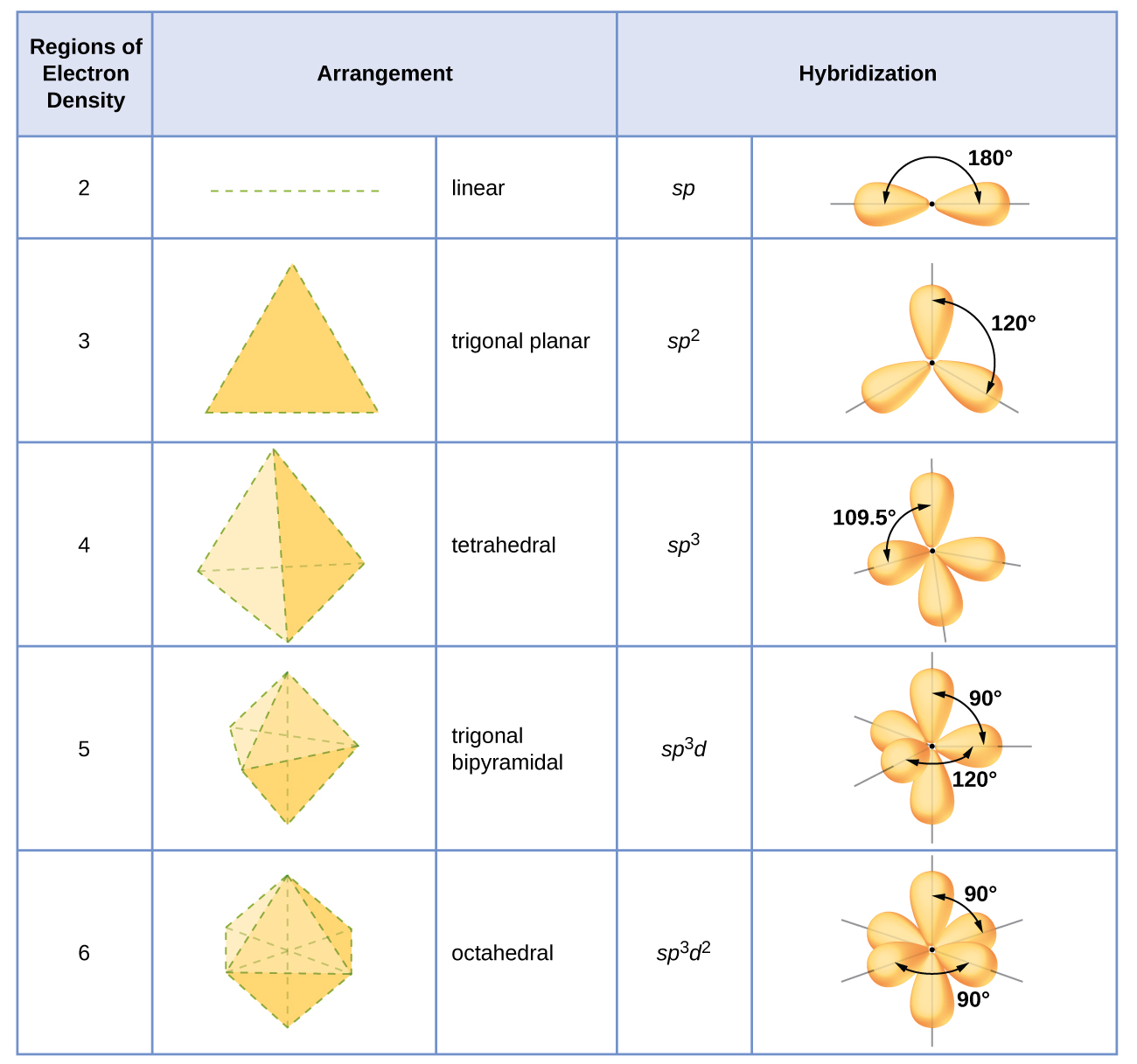
8.2 Hybrid Atomic Orbitals Chemistry LibreTexts
Pi Bonding Will Occur When There Is An.
This Organic Chemistry Video Tutorial Explains The Hybridization Of Atomic Orbitals.
Web By Hybridizing Its 2 S And 2 P Orbitals, It Can Form Four Sp3 Hybridized Orbitals That Are Equal In Energy.
It Discusses How To Determine The.
Related Post: