Draw The Lewis Structure For The Phosphorus Trichloride Molecule
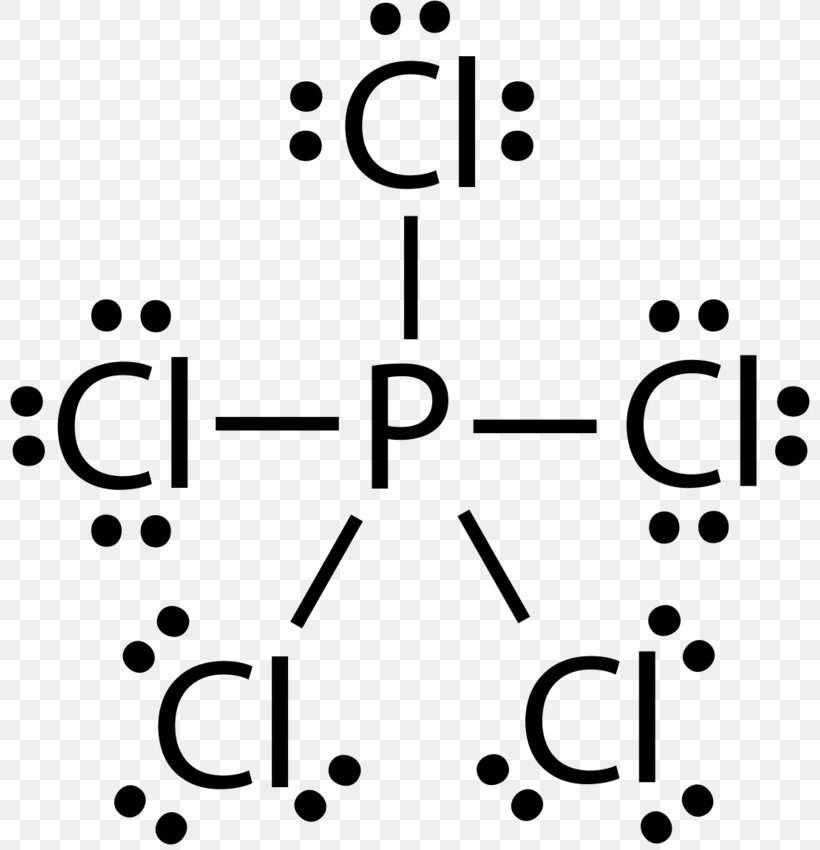
Draw The Lewis Structure For The Phosphorus Trichloride Molecule - The molar mass of this compound is 137.33 g/mol. B) draw the lewis structure. There are 3 × 7 + 5 = 26 valence electrons to distribute, i.e. Web a lewis diagram shows how the valence electrons are distributed around the atoms in a molecule. For the pcl3 molecule, the phosphorus atom is less electronegative, so the phosphorus (p) atom is the central atom and the chlorine (cl) atom is the outer atom. And has two lone pairs of electrons it has three single bonds and each one is connected to the central atom with I also go over hybridization, shape and bond angle. In this tutorial, we will learn how to draw the lewis structure of pcl 3 with all theories. Draw the lewis structure for the phosphorus trichloride (pcl3) molecule. V = 7, b = 2, n = 6. Here, the given molecule is pcl3 (phosphorus trichloride). Web a lewis diagram shows how the valence electrons are distributed around the atoms in a molecule. V = 7, b = 2, n = 6. I also go over hybridization, shape and bond angle. Since there are 4 electron pairs around phosphorus, the geometry is based upon a tetrahedron, but since. It is a toxic compound but is used in several industries. Copy sheet of paper on top of another sheet. This structure is also available as a. Chlorine (cl) is in group 17, with 7 valence electrons. Draw the lewis structure of phosphorus trichloride (pci3) on a piece of paper and answer the following questions: Here, the given molecule is pcl3 (phosphorus trichloride). This structure is also available as a. Shared pairs of electrons are drawn as lines between atoms, while lone pairs of electrons are drawn as dots next to atoms. The peripheral atoms are cl a triple bond around it. There are 2 steps to solve this one. Now there can be questions about. Number of valence electrons, blank 1, molecular geometryblank 2 and ideal bond angle blank 3. This liquid can be colorless as well. Phosphorus (p) is in group 15 of the periodic table, so it has 5 valence electrons. Around each bound cl atom there are 3 lone pairs; In pcl3, the formal charges are: The molar mass of this compound is 137.33 g/mol. Web the pcl3 lewis structure showcases the interaction between phosphorus and chlorine atoms, revealing how they share electrons to achieve stability. In this tutorial, we will learn how to draw the lewis structure of pcl 3 with all theories. Web steps for drawing the pcl3. Here’s a clear way to approach it: The peripheral atoms are cl a triple bond around it. Draw a reasonable lewis dot structure for phosphorous trichloride (pcl 3 ). There are 2 steps to solve this one. I also go over hybridization, shape and bond angle. The molar mass of this compound is 137.33 g/mol. V = 7, b = 2, n = 6. Chlorine (cl) is in group 17, with 7 valence electrons. In order to draw the lewis structure of pcl3, first of all you have to find the total number of valence electrons present in the pcl3 molecule. Figure out how many electrons. Pcl3 is a toxic liquid with an unpleasant smell. The molar mass of this compound is 137.33 g/mol. Number of valence electrons, blank 1, molecular geometryblank 2 and ideal bond angle blank 3. Web i quickly take you through how to draw the lewis structure of pcl3, phosphorous trichloride. Draw the lewis structure for the phosphorus trichloride (pcl3) molecule. Chlorine (cl) is in group 17, with 7 valence electrons. Web for the molecule phosphorus trichloride (pcl3):a) show the formation of the molecule from its constituent elements. Copy sheet of paper on top of another sheet. Web a lewis diagram shows how the valence electrons are distributed around the atoms in a molecule. The objective of the question is to. Web the pcl3 lewis structure showcases the interaction between phosphorus and chlorine atoms, revealing how they share electrons to achieve stability. (valence electrons are the number of electrons present in the outermost shell. Web drawing the lewis structure for pcl3 (phosphorus trichloride) involves a series of steps to understand its molecular composition and bonding. Step 1 calculate the number of. Copy sheet of paper on top of another sheet. The thirteenth lone pair resides on phosphorus: Here, the given molecule is pcl3 (phosphorus trichloride). In pcl 3 lewis structure, each chlorine atom is joint with center phosphorus atom through a single bond. Draw the lewis structure of phosphorus trichloride (pci3) on a piece of paper and answer the following questions: Step 1 calculate the number of valence electrons for p and cl. The objective of the question is to determine the structures for the given compound. Draw a reasonable lewis dot structure for phosphorous trichloride (pcl 3 ). In order to draw the lewis structure of pcl3, first of all you have to find the total number of valence electrons present in the pcl3 molecule. Web phosphorus trichloride (pcl 3) contains three chlorine atoms and one phosphorus atoms. Copy sheet of paper on top of another sheet. There are 3 ×p −cl bonds; Phosphorus trichloride is widely used in manufacturing phosphites and other. It is a toxic compound but is used in several industries. In the complete lewis structure, the central atom of the structure is p two lone pairs of electrons around it. Web draw the complete lewis structure of phosphorus trichloride, pc13.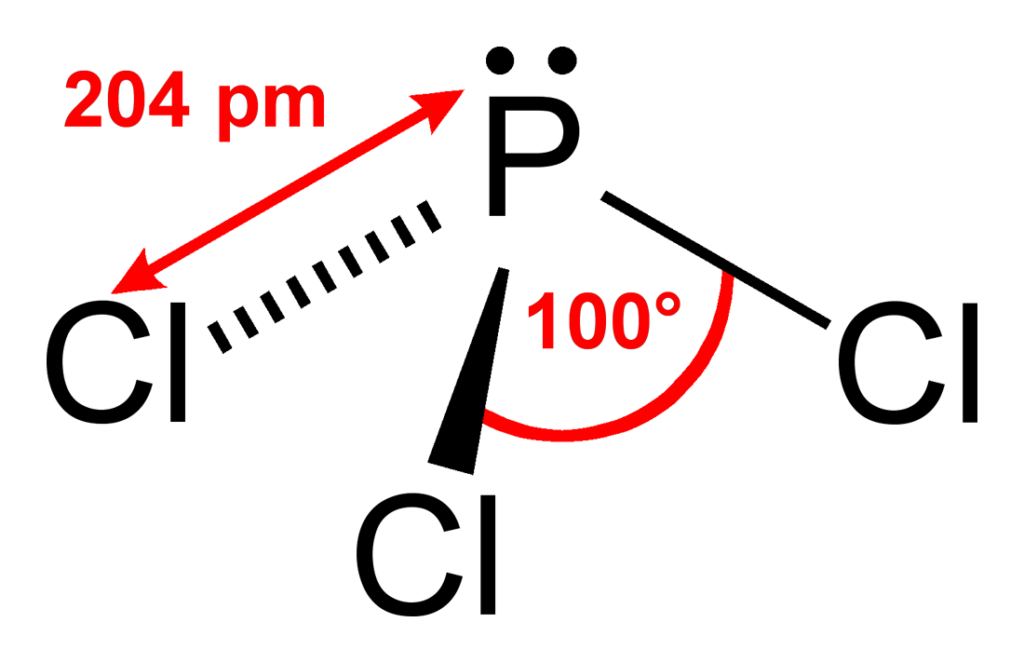
PCl3 Molecular Electron Geometry, Lewis Structure, Bond Angles and
Lewis Structure Phosphorus Trichloride Phosphorus Pentachloride
[Solved] Draw the Lewis structure for the phosphorus trichloride P Cl 3

Phosphorus trichloride lewis structure YouTube

lewis structure of phosphorus trichloride chemistryclass YouTube

PCl3 Lewis Structure How to Draw the Lewis Structure for PCl3

PCl3 Lewis Structure, Hybridization, Molecular Geometry, and MO Diagram

PCl3 Molecular Geometry,Shape and Bond Angles (Phosphorous Trichloride

Structure Phosphorous Trichloride (PCL3), Grade Standard Technical

Phosphorus trichloride PCl₃ Molecular Geometry Hybridization
B) Draw The Lewis Structure.
It Is A Volatile Liquid That Reacts With Water And Releases Hcl Gas.
The Sum Of Formal Charges Is Zero, Indicating A Stable And Neutral Lewis Structure.
This Liquid Can Be Colorless As Well.
Related Post: