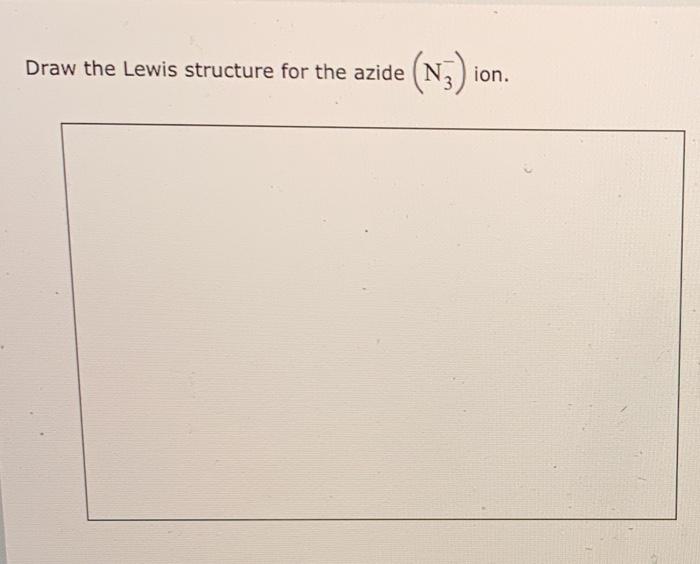
Draw The Lewis Structure For The Azide Ion
Draw The Lewis Structure For The Azide Ion - N3 lewis structure, hybridization, molecular structure, bond angle and shape. Web 70 more lewis dot structures. Web the lewis structure of the azide ion, [n₃]−, can be drawn by placing the three nitrogen atoms on the grid and connecting them with double bonds. It has 5 valence electrons. Sodium azide is found in air bags of automobiles. I also go over hybridization, shape and bond angles. Web the following procedure will give you the correct lewis structure for any molecule or polyatomic ion that has one central atom. You'll get a detailed solution from a subject matter. Web what is the lewis structure for the most stable azide ion, n − 3? This problem has been solved! The vsepr model predicts a shape that is linear. N3 lewis structure, hybridization, molecular structure, bond angle and shape. Web explore the formula and structure of the azide ion, a common component of explosives and propellants. It is a chemical formula for the azide ion. Draw the lewis structure for the azide (n3) ion. It has 5 valence electrons. Sodium azide is found in air bags of automobiles. Web when forming a lewis symbol for an ion, the chemical symbol is surrounded by dots that are used to represent valence electrons, and the whole structure is placed in square. The central n atom carries a negative charge. Web the following procedure will give you. This problem has been solved! It has 5 valence electrons. A video explanation of how to draw the lewis dot structure for the azide ion, along with information about the compound including. N3 lewis structure, hybridization, molecular structure, bond angle and shape. Nitrogen ordinarily has five electrons in its valence; N3 lewis structure, hybridization, molecular structure, bond angle and shape. Determine the total number of valence. You'll get a detailed solution from a subject matter. It is a chemical formula for the azide ion. Draw the lewis structure for the azide (n3) ion. Draw the lewis structure for the azide (n3) ion. This problem has been solved! Web what is the lewis structure for the most stable azide ion, n − 3? Web the lewis structure depicts these double bonds and a lone pair of electrons on each terminal n atom. The vsepr model predicts a shape that is linear. Web the following procedure will give you the correct lewis structure for any molecule or polyatomic ion that has one central atom. This problem has been solved! Determine the total number of valence. The vsepr model predicts a shape that is linear. Web when forming a lewis symbol for an ion, the chemical symbol is surrounded by dots that are. This problem has been solved! Web 70 more lewis dot structures. Nitrogen is in group 5, also called 15, on the periodic table. See how the three nitrogen atoms are bonded in this interactive 3d model. Draw the lewis structure for the azide (n3) ion. The vsepr model predicts a shape that is linear. I also go over hybridization, shape and bond angles. The central n atom carries a negative charge. A video explanation of how to draw the lewis dot structure for the azide ion, along with information about the compound including. Web the lewis structure depicts these double bonds and a lone pair. Azide does have resonance structures. Web the following procedure will give you the correct lewis structure for any molecule or polyatomic ion that has one central atom. A video explanation of how to draw the lewis dot structure for the azide ion, along with information about the compound including. You'll get a detailed solution from a subject matter. See how. Web the following procedure will give you the correct lewis structure for any molecule or polyatomic ion that has one central atom. Nitrogen ordinarily has five electrons in its valence; Determine the total number of valence. Azide does have resonance structures. Nitrogen is in group 5, also called 15, on the periodic table. Chemistry covalent bonds drawing lewis structures. The ion is made up of three nitrogen. Web 70 more lewis dot structures. The vsepr model predicts a shape that is linear. A video explanation of how to draw the lewis dot structure for the azide ion, along with information about the compound including. It is a chemical formula for the azide ion. This problem has been solved! It has 5 valence electrons. Draw the lewis structure for the azide (n3) ion. Web the lewis structure of the azide ion, [n₃]−, can be drawn by placing the three nitrogen atoms on the grid and connecting them with double bonds. N3 lewis structure, hybridization, molecular structure, bond angle and shape. Web the following procedure will give you the correct lewis structure for any molecule or polyatomic ion that has one central atom. Web the lewis structure depicts these double bonds and a lone pair of electrons on each terminal n atom. Nitrogen ordinarily has five electrons in its valence; You'll get a detailed solution from a subject matter. See how the three nitrogen atoms are bonded in this interactive 3d model.
Day03 4 Azide Ion (N3) & N2O4 Resonance & Avg Bond Order YouTube
Solved Draw the Lewis structure for the azide (N3) ion.
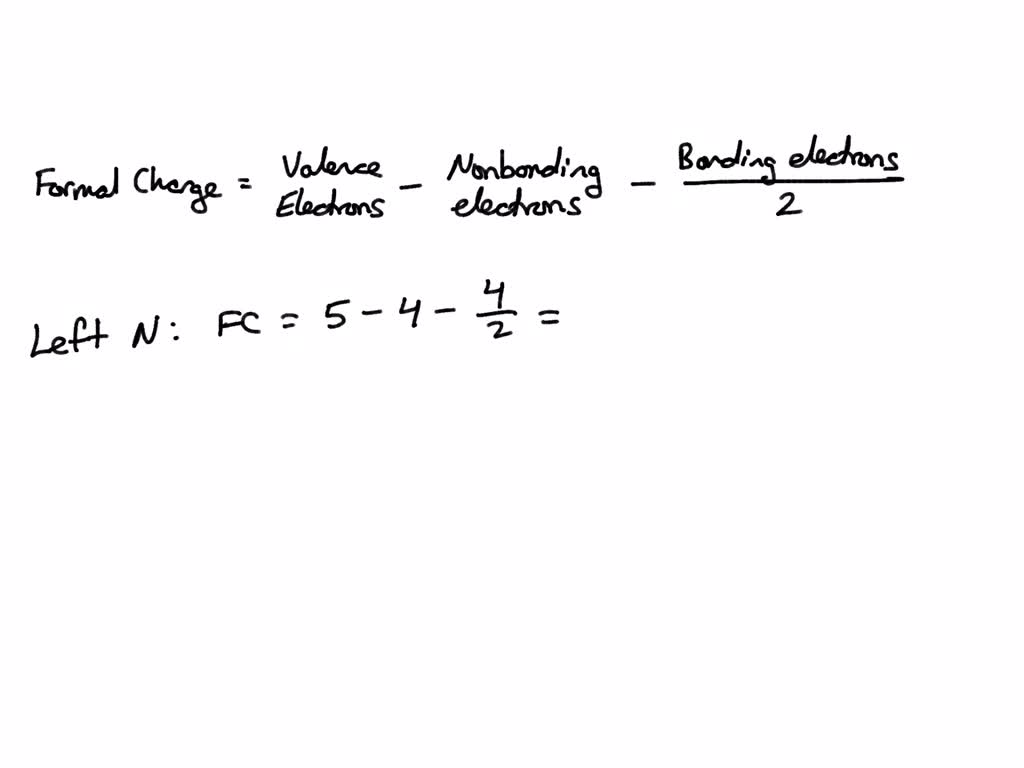
SOLVED Draw the Lewis structure of the azide ion, N3, and calculate
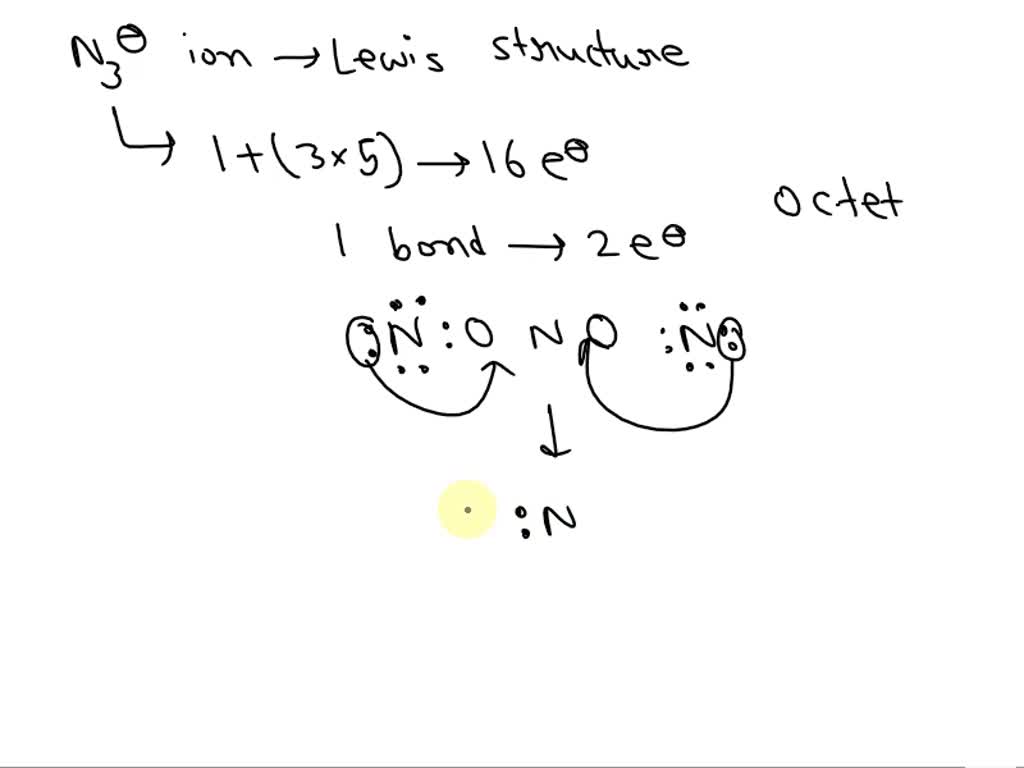
SOLVED Draw the Lewis structure of azide ion, Ns. (6 pts.)

N3 (Azide Ion) Molecular Geometry, Bond Angles & Electron Geometry
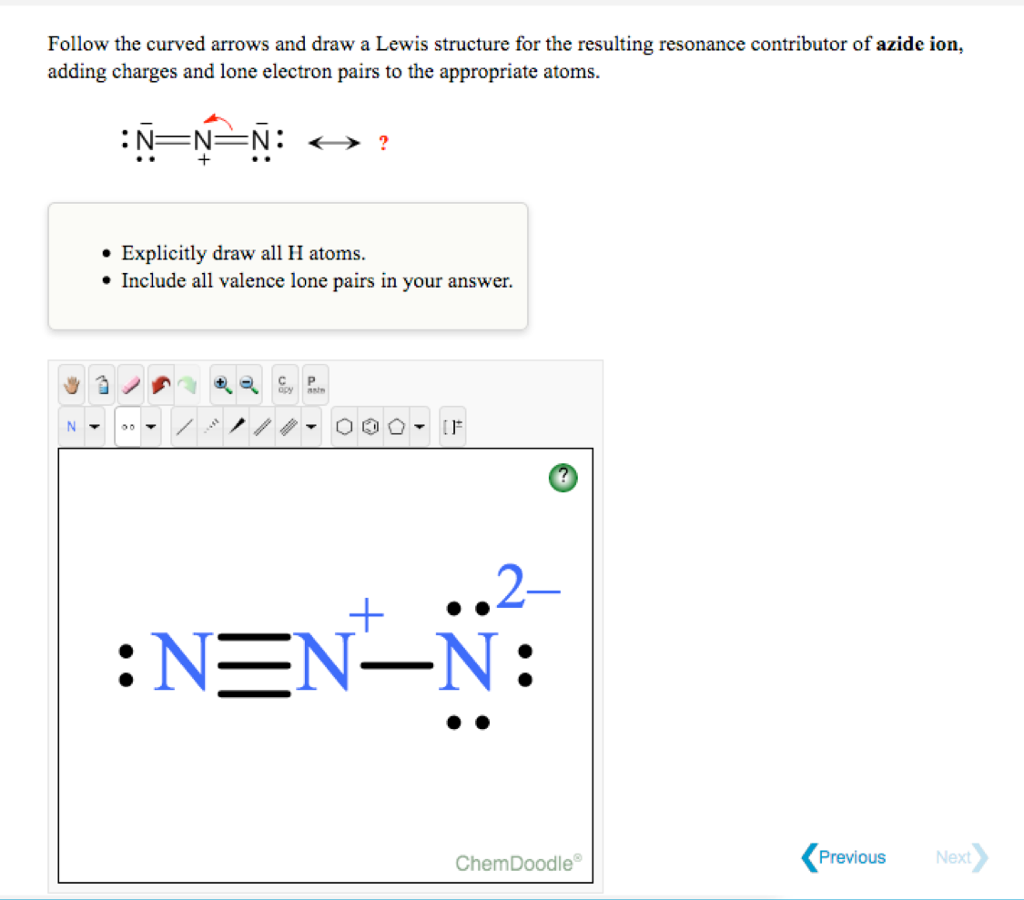
Solved Follow the curved arrows and draw a Lewis structure

Lewis Structure for N3 Azide ion YouTube

Molecular Orbitals Azide Anion
Solved Draw the Lewis structure for the azide (N, ion. Ć n

Draw the structure of azide ion \( \mathrm{P} \) YouTube
The Azide Ion Is A.
Determine The Total Number Of Valence.
Web What Is The Lewis Structure For The Most Stable Azide Ion, N − 3?
Azide Does Have Resonance Structures.
Related Post: