Draw The Lewis Structure For No3
Draw The Lewis Structure For No3 - If you chose to draw the electron configuration per element, you will have something like this: So, you can see the bond order decreases as the number of oxygens in these nok± n compounds increases. Nitrogen has 5 valence electrons, and each oxygen atom has 6 valence electrons. N2 (g)+3h2 (g)→2nh3 (g) buy. I also go over the resonance, hybridization, shape and bond angle. Calculate the total number of valence electrons. It is helpful if you: You will every fact of drawing lewis structures from this tutorial which will help you to draw more lewis structures in the future. Web the bond order for no− 2 is 3 electrons 2 bonding groups = 3/2 = 1.5. Web draw the lewis structures of all the molecules involved in the reaction: Draw a skeleton for the molecule which connects all atoms using only single bonds. 4 + (3 × 6) + 2 = 24 electrons. Web draw the lewis structure for no 3− , including the resonance structures if applicable. Web get the free lewis structure finder widget for your website, blog, wordpress, blogger, or igoogle. (valence electrons are the number. Show the determination of the total valence electrons that are in the lewis structure. Draw a skeleton for the molecule which connects all atoms using only single bonds. Find more chemistry widgets in wolfram|alpha. Web draw the lewis structure for no 3− , including the resonance structures if applicable. When we draw resonance structures, we convert lone pairs to bonds. Web construction of no3 lewis dot structure. 1 the nature of chemistry 2 chemical compounds 3 chemical reactions 4 energy and. If you chose to draw the electron configuration per element, you will have something like this: In simple molecules, the atom with the most available sites for bondng is usually placed central. In the ion no3, there is 1. Web draw the lewis structures of all the molecules involved in the reaction: 1 the nature of chemistry 2 chemical compounds 3 chemical reactions 4 energy and. I also go over the resonance, hybridization, shape and bond angle. The first step in drawing the lewis structure is to arrange the atoms in the molecule. Since nitrogen is less electronegative than. Determine the total number of valence electrons in the molecule. Hence, oxygen has 6 and nitrogen has 5 valence electrons in their outer shell. (valence electrons are the number of electrons present in the. (valence electrons are the electrons that are present in the outermost orbit of any. Since nitrogen is less electronegative than oxygen, it is placed in the. First determine the total number of valence electrons in the molecule. You can do this by (1) drawing the electron configuration per element or (2) consulting your periodic table. Web construction of no3 lewis dot structure. The bond order for no− 3 is 4 electrons 3 bonding groups = 4/3 ≈ 1.33. When we draw resonance structures, we convert lone. Hence, oxygen has 6 and nitrogen has 5 valence electrons in their outer shell. If you chose to draw the electron configuration per element, you will have something like this: The higher the bond order, the stronger the bond and thus the shorter the bond. In simple molecules, the atom with the most available sites for bondng is usually placed. Hence, oxygen has 6 and nitrogen has 5 valence electrons in their outer shell. This will be the sum of the group number a of all atoms plus the charge. Web draw the lewis structures of all the molecules involved in the reaction: This is in part to their high solubility in water. In simple molecules, the atom with the. Draw a skeleton for the molecule which connects all atoms using only single bonds. It is helpful if you: Web draw the lewis structure for no 3− , including the resonance structures if applicable. I also go over the resonance, hybridization, shape and bond angle. (valence electrons are the number of electrons present in the. Web draw the lewis structure for no 3− , including the resonance structures if applicable. Web an outline of how to detemine the best lewis structure for an example, no 3 is given below: If you chose to draw the electron configuration per element, you will have something like this: N2 (g)+3h2 (g)→2nh3 (g) buy. In simple molecules, the atom. 1s2 2s2 2p3 (5 outermost electrons) o (atomic number = 8. You will every fact of drawing lewis structures from this tutorial which will help you to draw more lewis structures in the future. N2 (g)+3h2 (g)→2nh3 (g) buy. Hence, oxygen has 6 and nitrogen has 5 valence electrons in their outer shell. This can be calculated by multiplying the valence electrons of each atom. The higher the bond order, the stronger the bond and thus the shorter the bond. This will be the sum of the group number a of all atoms plus the charge. (valence electrons are the electrons that are present in the outermost orbit of any. When we draw resonance structures, we convert lone pairs to bonds and bonds to lone pairs when it is possible. The bond order for no− 3 is 4 electrons 3 bonding groups = 4/3 ≈ 1.33. Web draw the lewis structures of all the molecules involved in the reaction: Nitrogen, belonging to group 15 of the periodic table, has five valence electrons, while oxygen, belonging to group 16, has six valence. Web draw the lewis structure for no 3− , including the resonance structures if applicable. Calculate the total number of valence electrons. Nitrogen has 5 valence electrons, and each oxygen atom has 6 valence electrons. Firstly, a nitrogen atom is drawn surrounded by three oxygen atoms in a.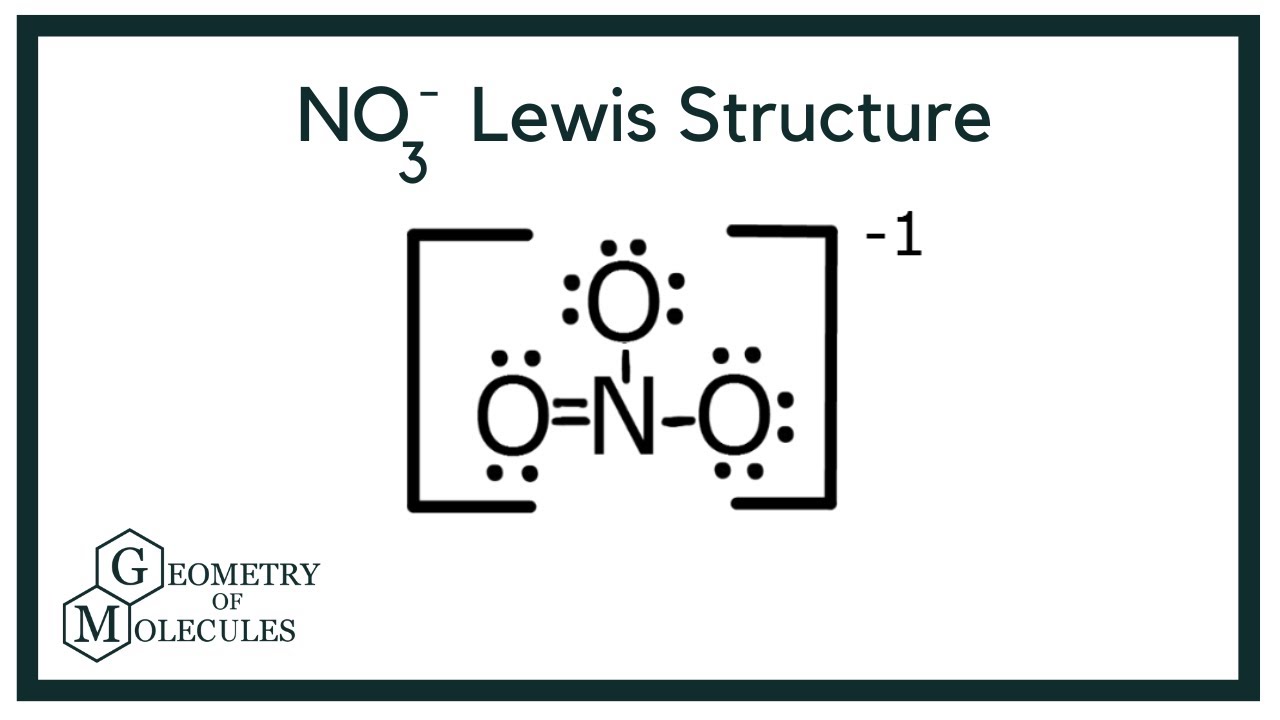
NO3 Lewis Structure Draw Lewis Dot Structure of Nitrate Ion YouTube

what is resonance?resonating structure of NO3 ion Brainly.in

Lewis Structure NO3 plus dipoles, shape, angles, resonance and formal
NO3 Lewis Structure How to Draw the Lewis Structure for NO3 YouTube

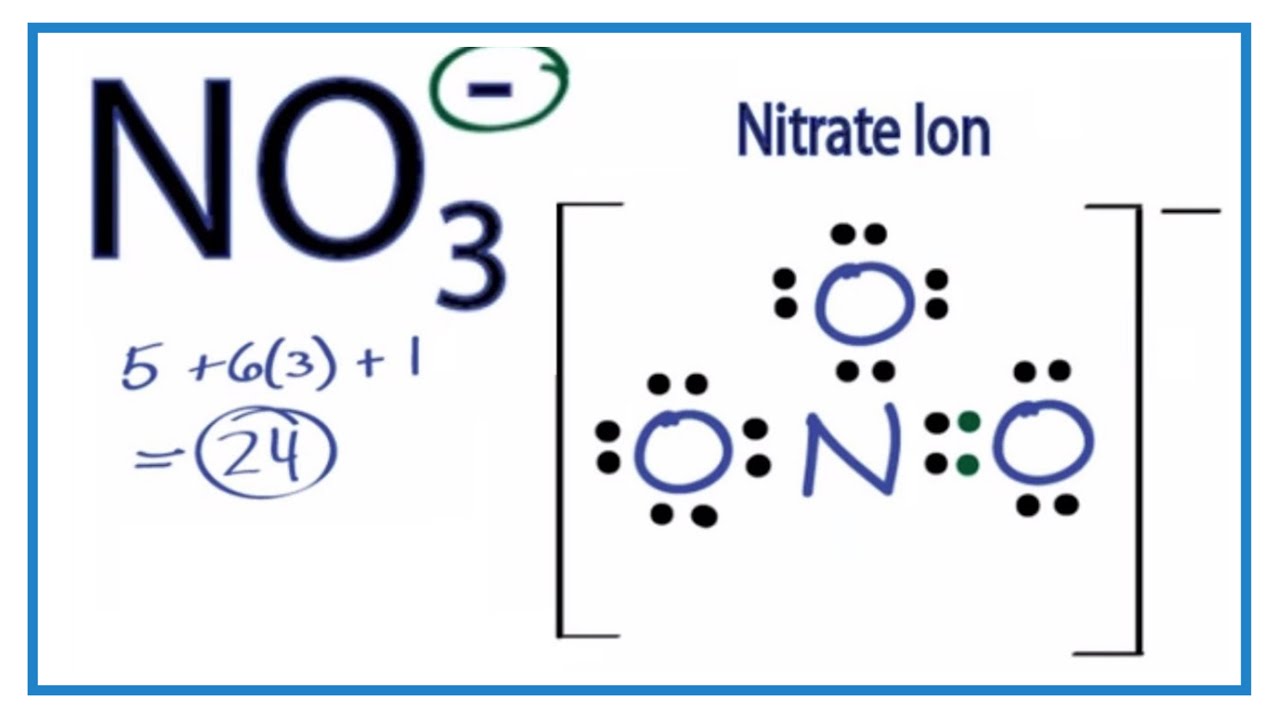
Lewis structure of NO3 (Nitrate ion)Draw the Lewis dot structure of
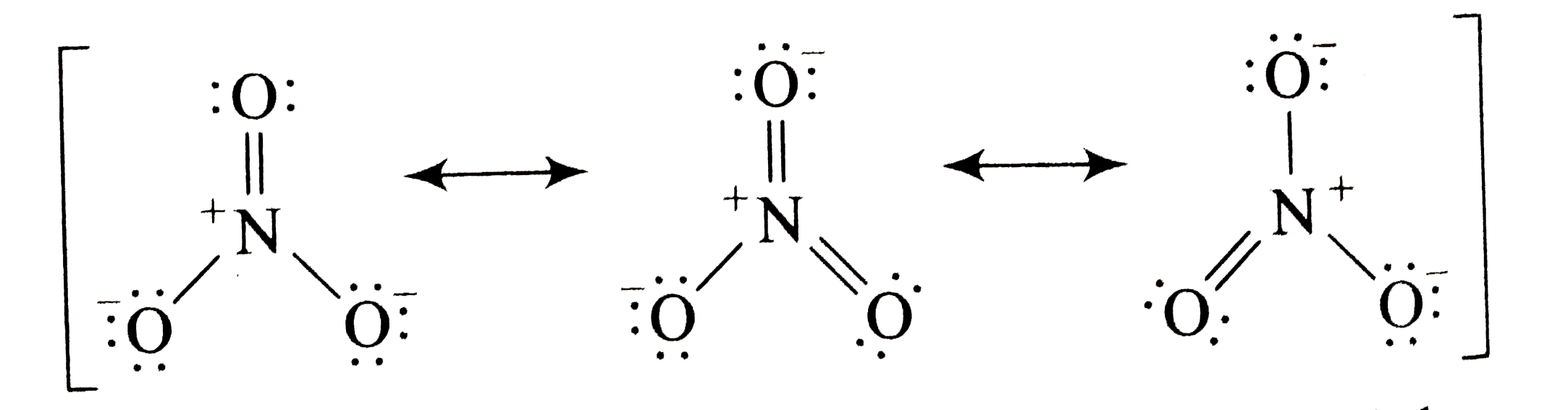
How many resonance structures can be drawn for the nitrate ion, NO3^(
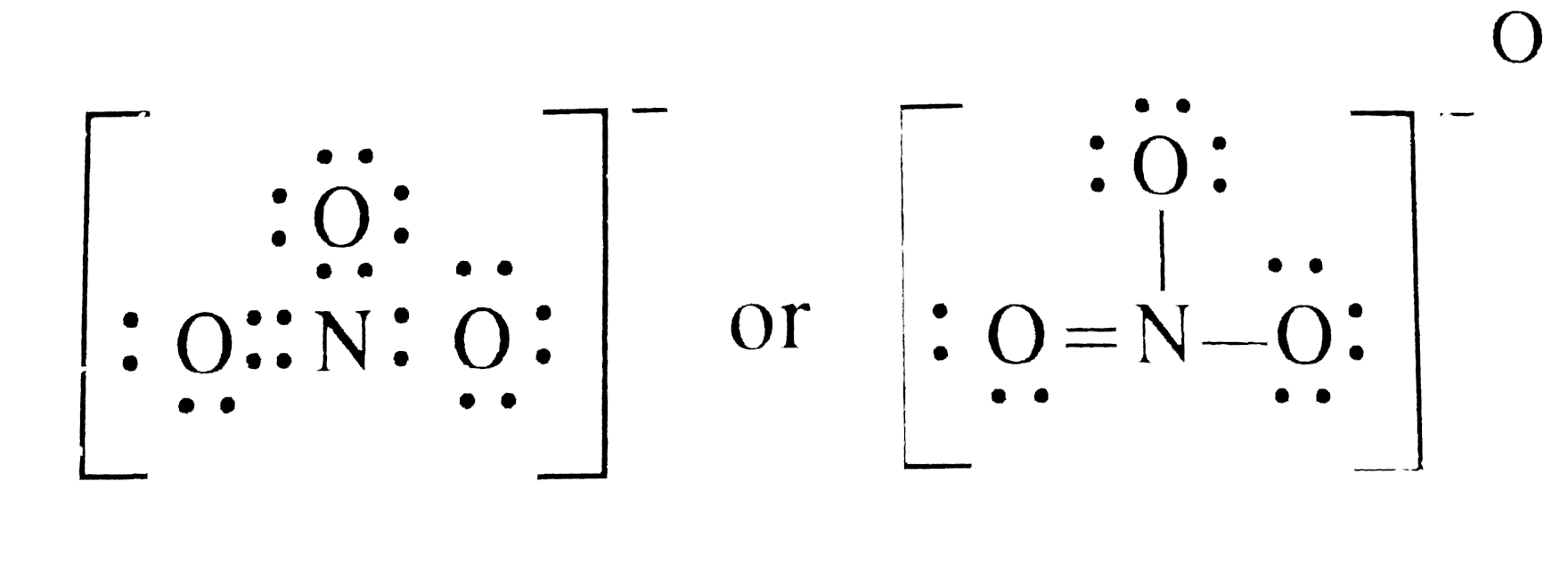
How To Draw The Lewis Dot Structure For No3 Nitrate Ion

NO3 Molecular Geometry / Shape and Bond Angles YouTube

Nitrate Ion Lewis Structure No3

NO3 Lewis Structure, Molecular Geometry, and Hybridization
1 The Nature Of Chemistry 2 Chemical Compounds 3 Chemical Reactions 4 Energy And.
(Valence Electrons Are The Number Of Electrons Present In The.
Web The Bond Order For No− 2 Is 3 Electrons 2 Bonding Groups = 3/2 = 1.5.
In Simple Molecules, The Atom With The Most Available Sites For Bondng Is Usually Placed Central.
Related Post: