Draw The Lewis Structure For Cocl2
Draw The Lewis Structure For Cocl2 - Here, the given molecule is cocl2. 8 + (6 × 7) = 50. In the lewis structure for cocl 2 there are a total of 24 valence electrons.; Include all lone pairs of electrons. Draw the lewis dot structure for the molecule by placing atoms on the grid and connecting them with bonds. Find the total valence electrons in cocl2 molecule. Web we can draw the lewis structure of any covalent molecule by following the six steps discussed earlier. The lewis electron structure is drawn within brackets as is customary for a molecular ion, with the overall charge indicated outside the brackets, and the bonding pair of electrons is indicated by a solid line. (assign lone pairs, radical electrons, and atomic charges where appropriate.) calculate the electrons required (er), valence electrons (ve), and shared pairs (sp). In the cocl 2 lewis structure, carbon is less electron electronegative than oxygen and goes in the center of the lewis structure (note that hydrogen atoms always go on the outside).; In the cocl 2 lewis structure, carbon is less electron electronegative than oxygen and goes in the center of the lewis structure (note that hydrogen atoms always go on the outside).; Draw the lewis structure of phosgene, cocl2. Step 2) attach the atoms to each other using single bonds (“draw the skeleton structure”) step 3) add electrons to all outer. (valence electrons are the number of electrons present. Web the formal charges being 0 for all of the atoms in the cocl 2 molecule tells us that the lewis dot structure presented above is stable. Web drawing the lewis structure for cocl 2. Calculate the total valence electrons in the molecule. Include all lone pairs of electrons. Calculate the number of valence electrons: Web get the free lewis structure finder widget for your website, blog, wordpress, blogger, or igoogle. 8 + (2 × 7) = 22. 8 + (6 × 7) = 50. (valence electrons are the electrons that are present in. 1×6 = 6 total = 24 valence electrons. Draw the lewis structure of phosgene, cocl2. 8 + (2 × 7) = 22. You'll need to form a double bond. In order to find the total valence electrons in a cocl2 molecule, first of all you should know the valence electrons present in carbon atom, oxygen atom as well as chlorine. Here, the given molecule is cocl2. Web this problem has been solved! To determine the hybridization of cobalt. #1 draw a rough sketch of the structure #2 next, indicate lone pairs on the atoms #3 indicate formal charges on the atoms, if necessary #4 minimize formal charges by converting lone pairs of the atoms #5 repeat step 4 if necessary,. Alternatively a dot method can be used to draw the lewis structure. Web steps of drawing cocl2 lewis structure step 1: Web the formal charges being 0 for all of the atoms in the cocl 2 molecule tells us that the lewis dot structure presented above is stable. 8 + (2 × 7) = 22. Bent linear tetrahedral trigonal planar. Draw the lewis dot structure for the molecule by placing atoms on the grid and connecting them with bonds. Thus, the lewis structure of cocl 2 is an exception to the octet rule. The final answer must have this number of electrons‼! Step 2) attach the atoms to each other using single bonds (“draw the skeleton structure”) step 3) add. Phosgene is a colorless gaseous compound known as carbonyl chloride and has a molecular weight of 98.92 gram/mol. One uses math, the other puzzle pieces to give the three correct structure. One possible arrangement is as follows: Find the total valence electrons in cocl2 molecule. Calculate the number of valence electrons: Determine the lewis structure of the molecule. 1×6 = 6 total = 24 valence electrons. Calculate the number of valence electrons: Therefore, the lewis structure for the cocl 2 is represented as follows:. All atoms have formal charges equals to 0 in this structure. One possible arrangement is as follows: In the lewis structure for cocl 2 there are a total of 24 valence electrons.; Bent linear tetrahedral trigonal planar trigonal pyramidal what is the cl−c−cl bood angle?what is the molecular shape of cocl2 ? Carbon (c) has 4 valence electrons, oxygen (o) has 6, and each chlorine (cl) atom has 7. 8 +. Bent linear tetrahedral trigonal planar trigonal pyramidal what is the cl−c−cl bood angle?what is the molecular shape of cocl2 ? Web drawing the lewis structure for cocl 2. The final answer must have this number of electrons‼! All atoms have formal charges equals to 0 in this structure. Web steps of drawing cocl2 lewis structure step 1: View the full answer step 2. Calculate the total valence electrons in the molecule. In order to find the total valence electrons in a cocl2 molecule, first of all you should know the valence electrons present in carbon atom, oxygen atom as well as chlorine atom. Therefore, the lewis structure for the cocl 2 is represented as follows:. In the lewis structure for cocl 2 there are a total of 24 valence electrons.; Web the formal charges being 0 for all of the atoms in the cocl 2 molecule tells us that the lewis dot structure presented above is stable. For cocl 2, it is as shown below: 1×6 = 6 total = 24 valence electrons. (valence electrons are the electrons that are present in. Draw the lewis structure of phosgene, cocl2. A lewis diagram shows how the valence electrons are distributed around the atoms in a molecule.
Lewis Dot Structure For Cocl2
![The Lewis Structure of COCl2 [with free study guide and video]](https://www.aceorganicchem.com/blog/wp-content/uploads/2023/05/COCl2-lewis-structure.jpg)
The Lewis Structure of COCl2 [with free study guide and video]
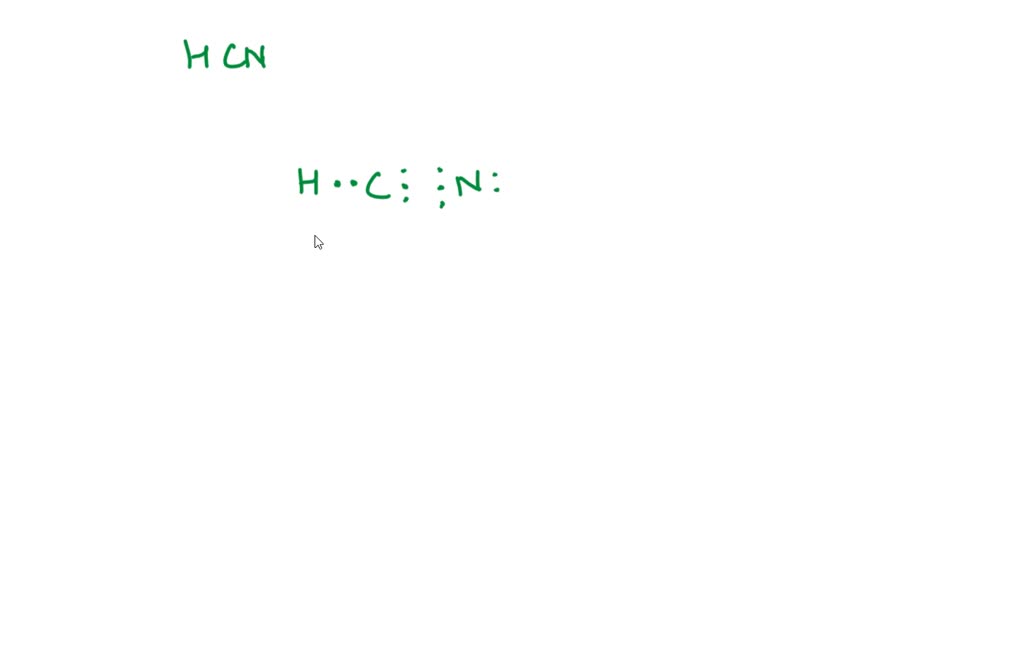
SOLVED Draw the Lewis structure for COCl2, including lone pairs.

COCl2 Lewis Structure How to Draw the Lewis Structure for COCl2 YouTube

COCl2 Lewis Structure (Phosgene) YouTube

Cocl2 Lewis Structure Shape Draw Easy
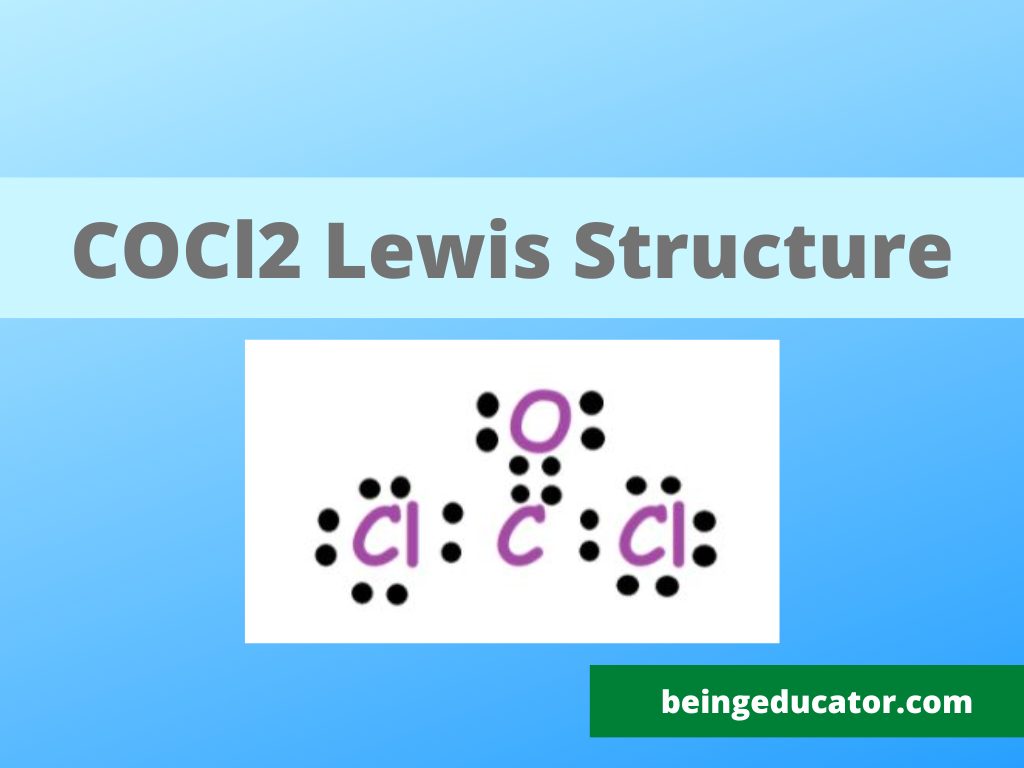
COCl2 Lewis Structure Hybridization Polarity Molecular Geometry

How to Calculate the Formal Charge of CoCl2 Sciencing

Cocl2 Lewis Dot Structure Draw Easy
Lewis Structure Of Cocl2
8 + (2 × 7) = 22.
You'll Need To Form A Double Bond.
Draw The Lewis Dot Structure For The Molecule By Placing Atoms On The Grid And Connecting Them With Bonds.
It Has A Boiling Point (B.p.) Of Around 8.3 0C.
Related Post: