Draw The Lewis Structure For Brf3
Draw The Lewis Structure For Brf3 - In order to draw the lewis structure of brf3, first of all you have to find the total number of valence electrons present in the brf3 molecule. Brf3 draw the lewis structure of brf3 and then determine the ideal bonding angle (s) of the central atom. Web here is what is needed: Web a video explanation of how to draw the lewis dot structure for bromine trifluoride , along with information about the compound including formal charges, pola. Draw the lewis structure for brf with an arrow representing the dipole moment. In order to find the total valence electrons in a brf3 molecule, first of all you should know the valence electrons present in the bromine atom as well as fluorine atom. Drawing the lewis structure for brf 3. How many electron groups are present around the central atom and what is the electron group geometry? 4 + (3 × 6) + 2 = 24 electrons. The hybridization on the br is (sp, sp2, sp3, sp3d, sp3d2). Web in the brf3 lewis structure, there are three single bonds around the bromine atom, with three fluorine atoms attached to it. The molecule is (choose one: Br is the central atom, connected to each f atom by a single bond. Draw a lewis structure for the following three molecules. Web study with quizlet and memorize flashcards containing terms like. There are a total of 28 valence electrons for the brf 3 lewis structure. 3 lone electron pairs will surround each f. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Step 2) attach the atoms to each other using single bonds (“draw the skeleton structure”) step 3) add electrons to all outer. Now, as there are three fluorine atoms, the electrons in its outer shell. There are 4 steps to solve this one. Draw the lewis structure for brf with an arrow representing the dipole moment. 4 + (3 × 6) + 2 = 24 electrons. The bromine atom (br) and each fluorine atom (f) are individually connected by a single bond. In order to find the total valence electrons in a brf3 molecule, first of all you should know the valence electrons present in the bromine atom as well as fluorine atom. The lewis structure for brf a 3 molecule can be drawn by identifying the electronic configuration and valen. Draw the best lewis dot structure for brf3 in the correct. The cl atom is hybridized. For the brf 3 lewis structure, you'll need to put more than eight valence electrons on the bromine atom.; Now, as there are three fluorine atoms, the electrons in its outer shell. Find the total valence electrons in brf3 molecule. The hybridization on the br is (sp, sp2, sp3, sp3d, sp3d2). For the brf 3 lewis structure, calculate the total number of valence electrons for the brf 3 molecule. In order to draw the lewis structure of brf3, first of all you have to find the total number of valence electrons present in the brf3 molecule. Bromine and fluorine all each bring 7 valence e. The bromine atom (br) and each. Web in lewis structure formation, we have to check whether all the atoms have their least possible formal charge values. The cl atom is hybridized. Use these steps to correctly draw the brf 3 lewis structure: What is the molecular geometry and ideal bond angles? Please show the steps you went through to obtain your final structure. After determining how many valence electrons there are in brf 3, place them around the. Here, the given molecule is brf3 (bromine trifluoride). For the brf 3 lewis structure, calculate the total number of valence electrons for the brf 3 molecule. Brf3 draw the lewis structure of brf3 and then determine the ideal bonding angle (s) of the central atom.. Draw the molecule by placing atoms on the grid and connecting them with bonds. In order to find the total valence electrons in a brf3 molecule, first of all you should know the valence electrons present in the bromine atom as well as fluorine atom. Drawing the lewis structure for brf 3. 3 lone electron pairs will surround each f.. In the lewis structure for brf 3 there are a total of 28. The hybridization on the br is (sp, sp2, sp3, sp3d, sp3d2). For the brf 3 lewis structure, you'll need to put more than eight valence electrons on the bromine atom.; Now, as there are three fluorine atoms, the electrons in its outer shell. Draw the lewis structure. Bromine and fluorine all each bring 7 valence e. In the brf 3 lewis structure bromine (br) is the least electronegative atom and goes in the center of the lewis structure.; Draw the molecule by placing atoms on the grid and connecting them with bonds. The molecule is (choose one: Brf3 does not follow the octet rule. We can see that the three f atoms and the single br atom all have their formal charge value to be 0. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Each atom in this molecule has seven valence electrons, so you can keep seven dots around each atom in the compound. Web drawing the lewis structure for brf 3. After determining how many valence electrons there are in brf 3, place them around the. Draw a lewis structure for the following three molecules. The 3d lewis structure of brf3 can be visualized as a trigonal. Calculate the total number of valence electrons. #1 first draw a rough sketch #2 mark lone pairs on the atoms #3 calculate and mark formal charges on the atoms, if. The bromine atom (br) and each fluorine atom (f) are individually connected by a single bond. The final answer must have this number of electrons‼!
Lewis Structure of BrF3 (bromine trifluoride) YouTube

How to draw BrF3 Lewis Structure? 3
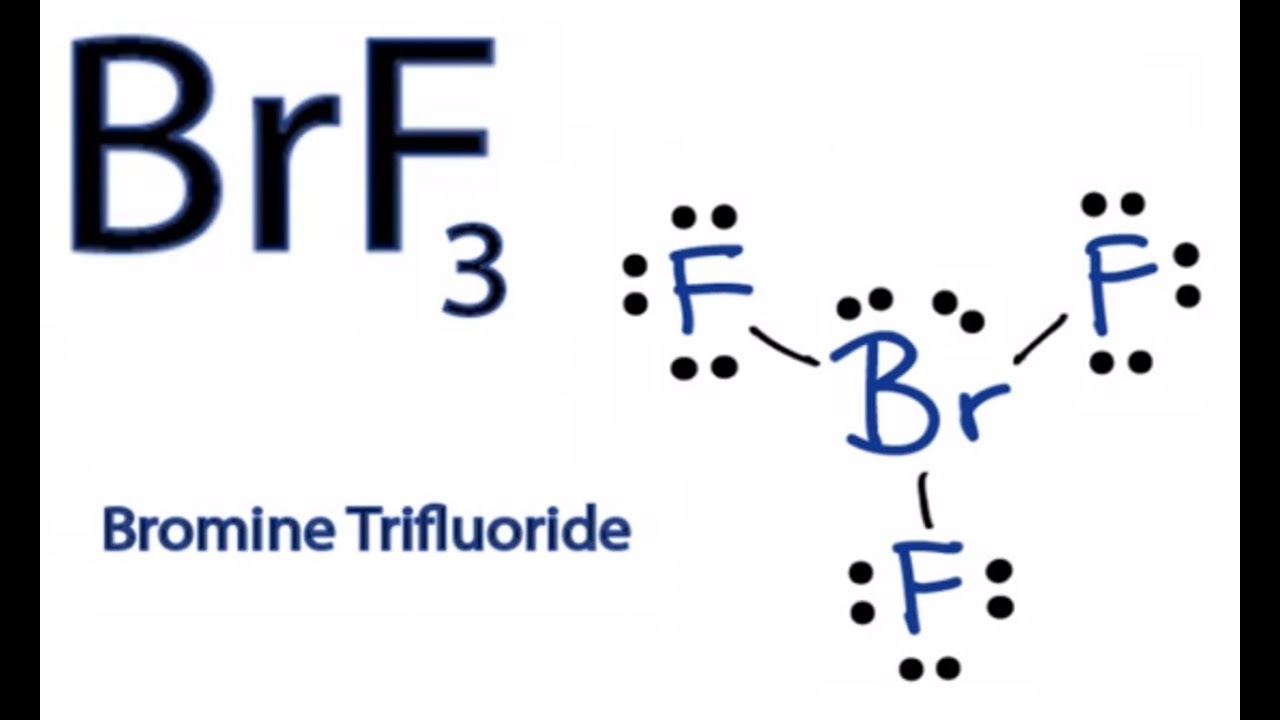
BrF3 Lewis Structure How to Draw the Lewis Structure for BrF3 YouTube
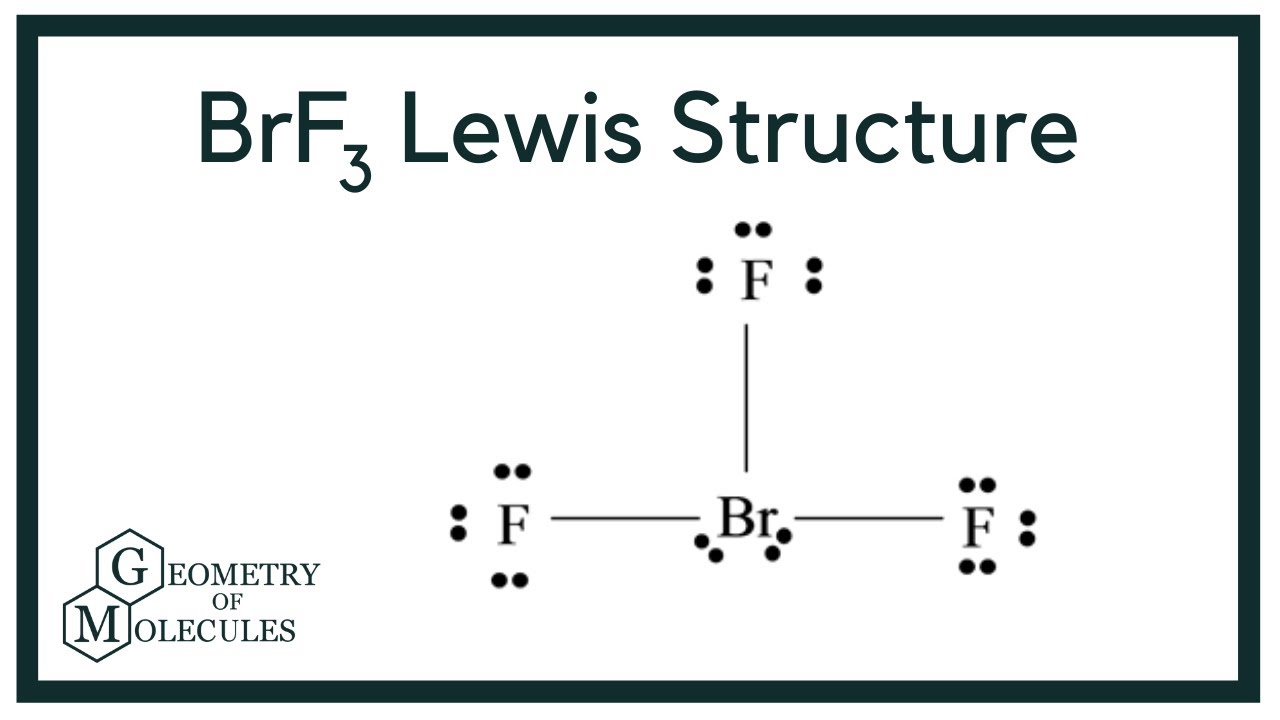
BrF3 Lewis Structure (Bromine Trifluoride) YouTube

Brf3 Lewis Structure Molecular Geometry Hybridization And Mo Diagram
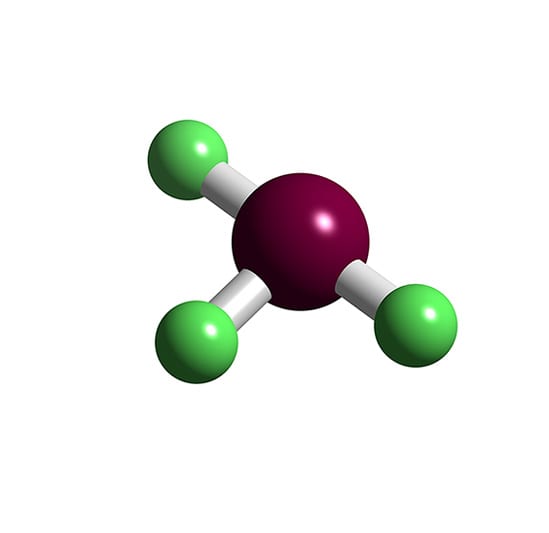
Brf3 Molecule

draw the lewis structure for brf3 in the window below and then decide

BrF3 Lewis Structure, Molecular Geometry, Hybridization, and MO Diagram

Estructura de Brf3 Lewis, características 13 datos que debe conocer

BrF3 Lewis Structure, Molecular Geometry, Hybridization, and MO Diagram
Please Show The Steps You Went Through To Obtain Your Final Structure.
Use These Steps To Correctly Draw The Brf 3 Lewis Structure:
What Is The Molecular Geometry And Ideal Bond Angles?
Include All Lone Pairs Of Electrons.
Related Post: