Draw The Lewis Structure For A Hydroxide Ion
Draw The Lewis Structure For A Hydroxide Ion - Web drawing a lewis structure is pretty simple! Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. • include all valence lone pairs in your answer. Become a study.com member to unlock this answer! • do not use the square brackets tool in your answer. The ch2o c h 2 o molecule. Make connection of bonding within all elements present in structure. • explicitly draw all h atoms. The oxygen and hydrogen are connected by a bond. Web the hydroxide ion must thus carry a single negative charge. Web the 2015 and 2020 rules focused on a specific design that employs hydroxide precipitation, sulfide precipitation (organosulfide), and iron coprecipitation to remove suspended solids and convert soluble metal ions to insoluble metal hydroxides or sulfides. Get the free lewis structure finder widget for your website, blog, wordpress, blogger, or igoogle. This problem has been solved! The following hydroxide ion. To understand how to do this, let’s use the example of the hydroxide ion: Draw the lewis structure for the water molecule.b. Web the hydroxide ion must thus carry a single negative charge. Including a single electron from the o−h bond, there are 9 electrons associated with the o nucleus and if there are 9 such electrons, the o atom. 1.6k views 2 years ago. Draw a complete structure for hydroxide ion, oh. Find more chemistry widgets in wolfram|alpha. When drawing the structure of an ion, be sure to add/subtract electrons to account for. In order to draw the lewis structure for a given ion, we must first determine how many valence electrons are involved. Web the hydroxide ion must thus carry a single negative charge. This problem has been solved! To understand how to do this, let’s use the example of the hydroxide ion: Calculate the total number of valence electrons in the molecule. For the hydroxide ion structure use the periodic table to find. This problem has been solved! Make connection of bonding within all elements present in structure. Suppose the structure of h 3 o + is required. Web the 2015 and 2020 rules focused on a specific design that employs hydroxide precipitation, sulfide precipitation (organosulfide), and iron coprecipitation to remove suspended solids and convert soluble metal ions to insoluble metal hydroxides or. Web chemistry questions and answers. 1.6k views 2 years ago. What is an example of a lewis structures practice problem? The following hydroxide ion contains one oxygen atom and one hydrogen atom. • include all nonzero formal charges. Draw a complete structure for hydroxide ion, oh. Find more chemistry widgets in wolfram|alpha. P aste ору c , # [ ] chemdoodle. Following are the points mentioned to draw the lewis structure of any molecule: Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. To understand how to do this, let’s use the example of the hydroxide ion: Following are the points mentioned to draw the lewis structure of any molecule: You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Get the free lewis structure finder widget for your website, blog, wordpress, blogger, or igoogle. Web the. Our discussion of ionic compounds was confined to monatomic ions and our discussion of covalent compounds was confined to neutral molecules. Web go ahead… i’ll wait for you to come back. Web the following procedure will give you the correct lewis structure for any molecule or polyatomic ion that has one central atom. Following are the points mentioned to draw. Become a study.com member to unlock this answer! The following hydroxide ion contains one oxygen atom and one hydrogen atom. • explicitly draw all h atoms. Draw the lewis structure for the water molecule.b. What is the lewis structure for so2? You'll get a detailed solution from a subject matter expert that helps you learn core concepts. The ocl− o c l − ion. Explicitly draw all h atoms. Central position is occupied by the element with lowest electronegativity. How do you draw the lewis structure for ionic compounds? What are lewis dot structures used for? Web the hydroxide ion must thus carry a single negative charge. This problem has been solved! Web drawing a lewis structure is pretty simple! Web the 2015 and 2020 rules focused on a specific design that employs hydroxide precipitation, sulfide precipitation (organosulfide), and iron coprecipitation to remove suspended solids and convert soluble metal ions to insoluble metal hydroxides or sulfides. That's the only way you can make this lewis structure work. This is done in the same way as for neutral ions, except that when you’re done, you need to change the number of electrons to compensate for the charge on the ion. The ch2o c h 2 o molecule. Find the number of valence electrons. The h2o h 2 o molecule. Write a lewis structure for the hydroxide ion.
The Lewis Structure of Hydroxide Ion (OH) YouTube

OH Lewis Structure Lewis Dot Structure for OHHydroxide Ion Lewis

OH Lewis Structure Hydroxide Ion Lewis Dot Structure YouTube

How to Draw the Lewis Dot Structure for the Hydroxide ion YouTube
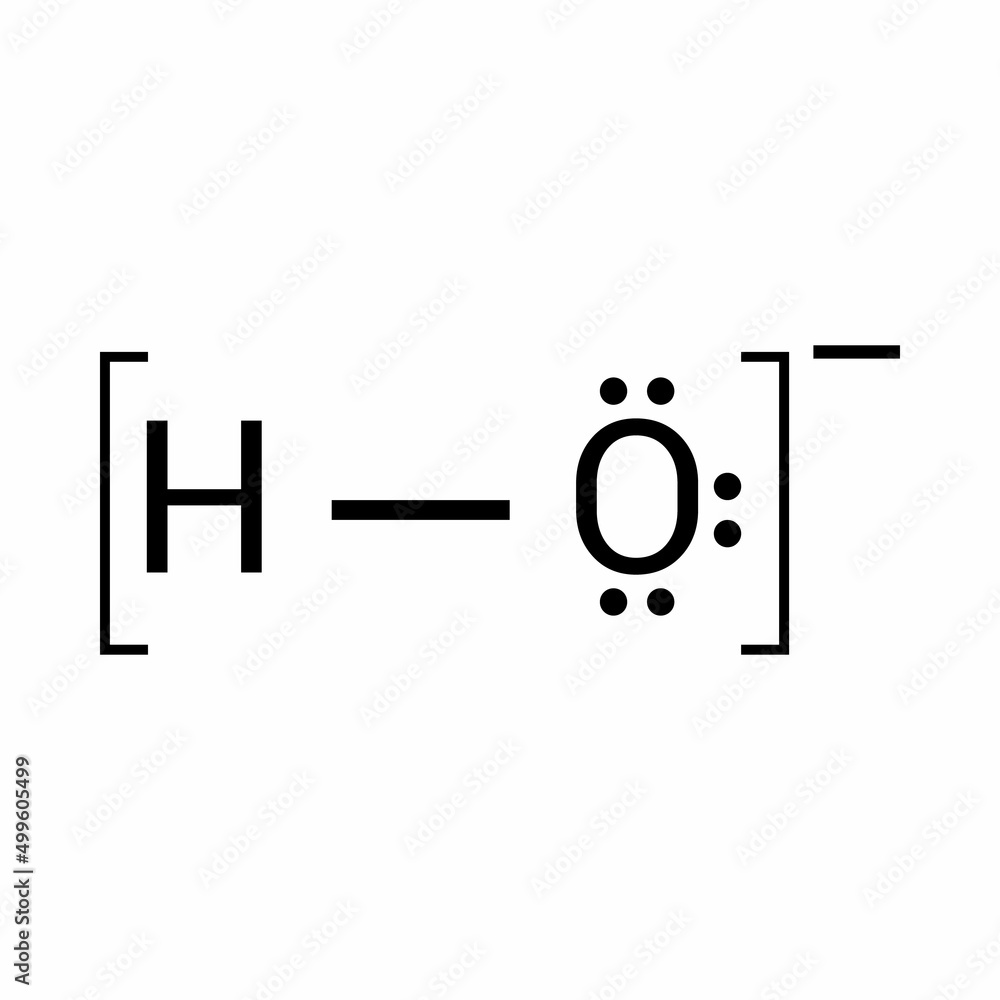
lewis structure of hydroxide ion Stock Vector Adobe Stock

How to Draw the Lewis Dot Structure for H+ (Hydrogen ion) YouTube

OH Lewis Structure How to Draw the Lewis Dot Structure for the

H2O Lewis Structure, Molecular Geometry, and Hybridization

A stepbystep explanation of how to draw the H3O+ Lewis Structure

Draw the electron dot structure of the hydroxide ion (OH^ Quizlet
Suppose The Structure Of H 3 O + Is Required.
Web This Widget Gets The Lewis Structure Of Chemical Compounds.
The Molecule Does Not Have A Formal Geometry Since It Is An Ion With A Full Negative Formal Charge On The O Atom Show More.
Get The Free Lewis Structure Finder Widget For Your Website, Blog, Wordpress, Blogger, Or Igoogle.
Related Post: