Draw The Lewis Dot Structure For H2S
Draw The Lewis Dot Structure For H2S - Note down a skeletal structure displaying a realistic bonding pattern by means of only the element symbols. Play quiz game > +1 vote. Web let's do the lewis structure for h2s: This is the lewis dot structure for h2s (hydrogen sulfide). Web to draw the lewis structure of an atom, write the symbol of the atom and draw dots around it to represent the valence electrons. Web draw the lewis structures for the following molecules and ions: Web draw the lewis dot structure of a given molecule or ion. The sulfur atom have 2 lone pairs. Draw the electron dot stucture of h 2s (hydrogen sulphide)? Web what is the lewis structure of hydrogen sulfide h2s? Check the octet rule (formal charges) step 6: #3 indicate formal charges on the atoms, if necessary. Web let's do the lewis structure for h2s: Web what is the lewis structure of hydrogen sulfide h2s? The sulfur atom (s) is at the center and it is surrounded by 2 hydrogen atoms (h). Answered jan 24, 2020 by surajkumar (64.8k points) selected jan 25, 2020 by nishu03. The rules for drawing lewis structures permit the replacement of the bond lines with two electrons. Valence electrons in hydrogen (h): Count total valence electrons for the molecule.2. Web draw the lewis structures for the following molecules and ions: These valence electrons are negatively charged and are attracted to the positively charged nucleus, made up of neutrons and protons. Web lewis dot structures, or lewis structures for short, are visuals that represent the outermost shell of electrons, also known as valence electrons, and possible covalent bonds within an atom or molecule. #1 draw a rough sketch of the structure.. The lewis structure of h2s is similar to h2s. For the h2s structure use the periodic table to find the total number of valence electrons. Steps of drawing h2s lewis structure. View best match solution instead. Only give reasonable results for covalent compounds and polyatomic ions of the main group (s and p block) elements, can not predict the structure. Determining the total valence electrons. Assess the stability of a structure by considering formal charges of atoms. These valence electrons are negatively charged and are attracted to the positively charged nucleus, made up of neutrons and protons. 40k views 2 years ago lewis structures. #1 draw a rough sketch of the structure. To accurately represent the h2s lewis structure, we need to calculate the total valence electrons. Count total valence electrons for the molecule.2. Draw the electron dot stucture of h 2s (hydrogen sulphide)? #1 draw a rough sketch of the structure. Hydrogen is satisfied with 2 electrons (1 bond), and sulfur is satisfied with 8 electrons (2 bonds and 2 lone. Play quiz game > +1 vote. Valence electrons in hydrogen (h): Hydrogen valence electron = 1. View best match solution instead. For the h2s structure use the periodic table to find the total number of valence electrons. Web lewis dot structures, or lewis structures for short, are visuals that represent the outermost shell of electrons, also known as valence electrons, and possible covalent bonds within an atom or molecule. For the h2s structure use the periodic table to find the total number of valence electrons. Web to properly draw the h 2 s lewis structure, follow these. 40k views 2 years ago lewis structures. Answered jan 24, 2020 by surajkumar (64.8k points) selected jan 25, 2020 by nishu03. These valence electrons are negatively charged and are attracted to the positively charged nucleus, made up of neutrons and protons. How to draw the dot structure for h2s; Find the total valence electrons in h2s molecule. Draw resonance structures of some molecules. Web lewis dot structures, or lewis structures for short, are visuals that represent the outermost shell of electrons, also known as valence electrons, and possible covalent bonds within an atom or molecule. Assess the stability of a structure by considering formal charges of atoms. Count the total number of valence electrons; Put the least. How to draw electron dot structures? The sulfur atom (s) is at the center and it is surrounded by 2 hydrogen atoms (h). Count the total number of valence electrons; Web the lewis dot structure for h2s. Find the total valence electrons in h2s molecule. Let’s break down each step in more detail. Put the least electronegative atom. Give examples for molecules and ions that do not follow the octet rule. Count total valence electrons for the molecule.2. This is the lewis dot structure for h2s (hydrogen sulfide). Stages to articulate the electron dot formula are stated beneath. Hydrogen is satisfied with 2 electrons (1 bond), and sulfur is satisfied with 8 electrons (2 bonds and 2 lone pairs). How to draw the dot structure for h2s; Plus sulfur is in group 6 or 16 on the periodic table, so it has 6 valence electrons. Web what is the lewis structure of hydrogen sulfide h2s? Draw the electron dot structure for h2s.
H2S Lewis Structure ,Valence Electrons ,Formal Charge,Polar or Nonpolar
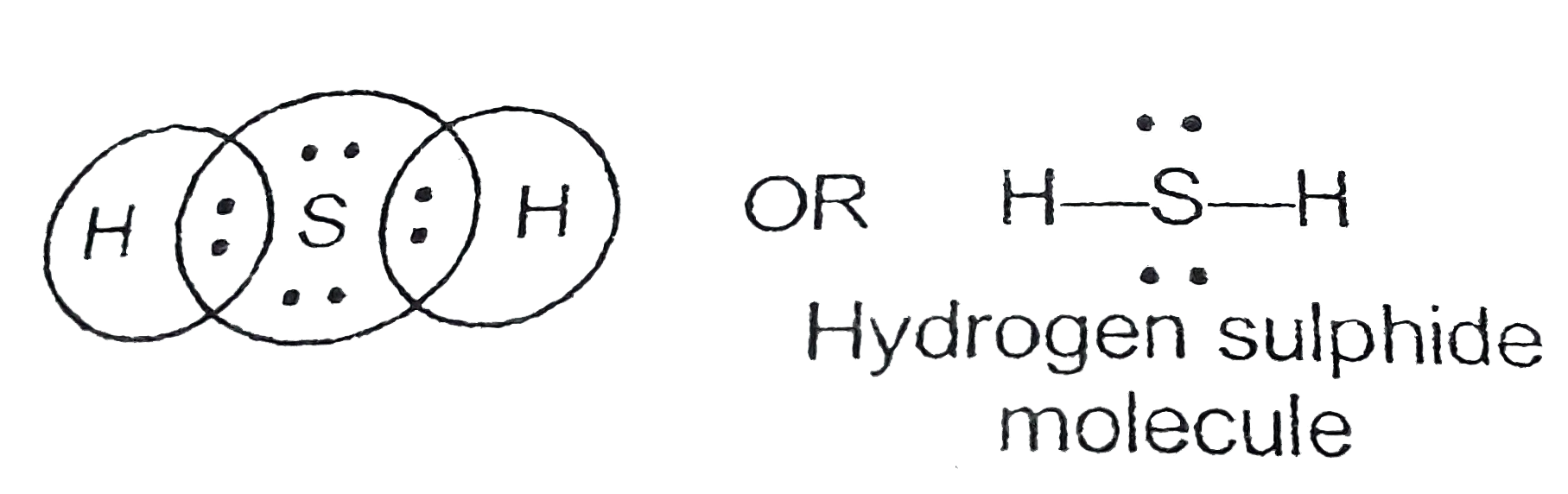
Draw the Lewis dot structure of Hydrogen sulphide molecule

H2S Lewis Structure, Molecular Geometry, Hybridization, and MO Diagram

Lewis Structure Hydrogen Sulfide H2s Stock Vector (Royalty Free

H2S Lewis Structure How to Draw the Dot Structure II lSCIENCE ll
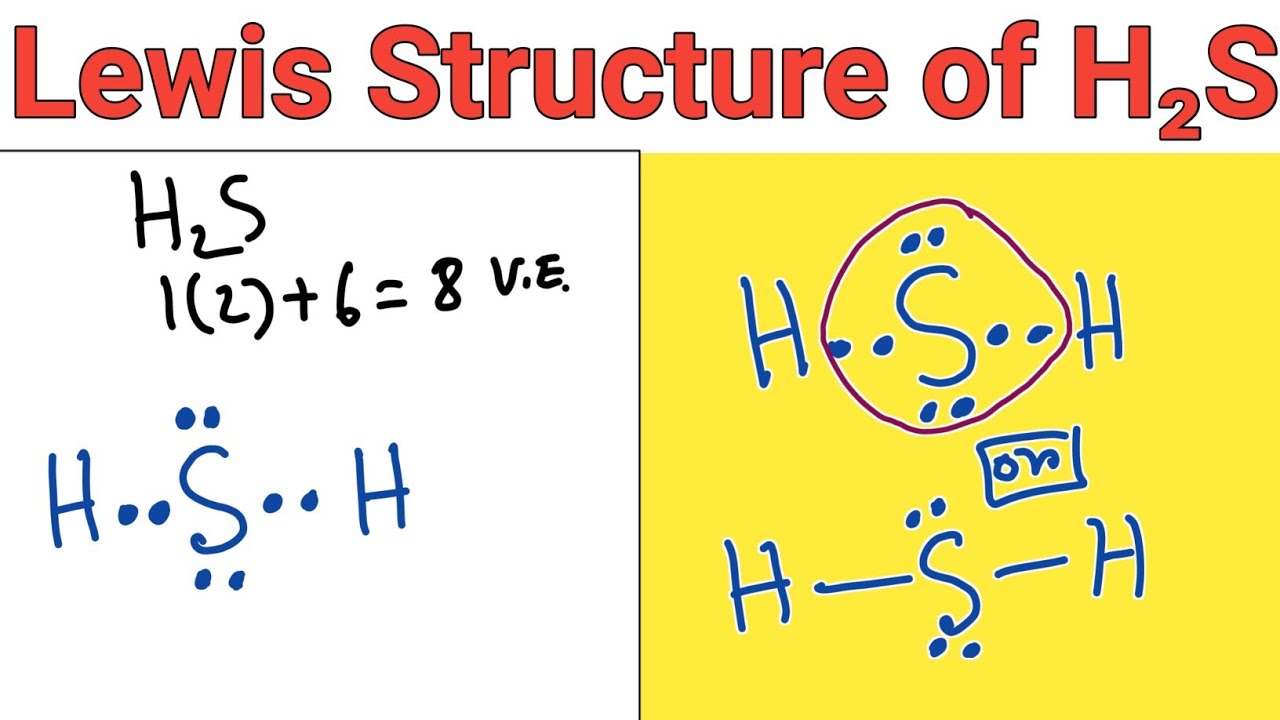
Lewis structure of H2S (Hydrogen sulphide) YouTube
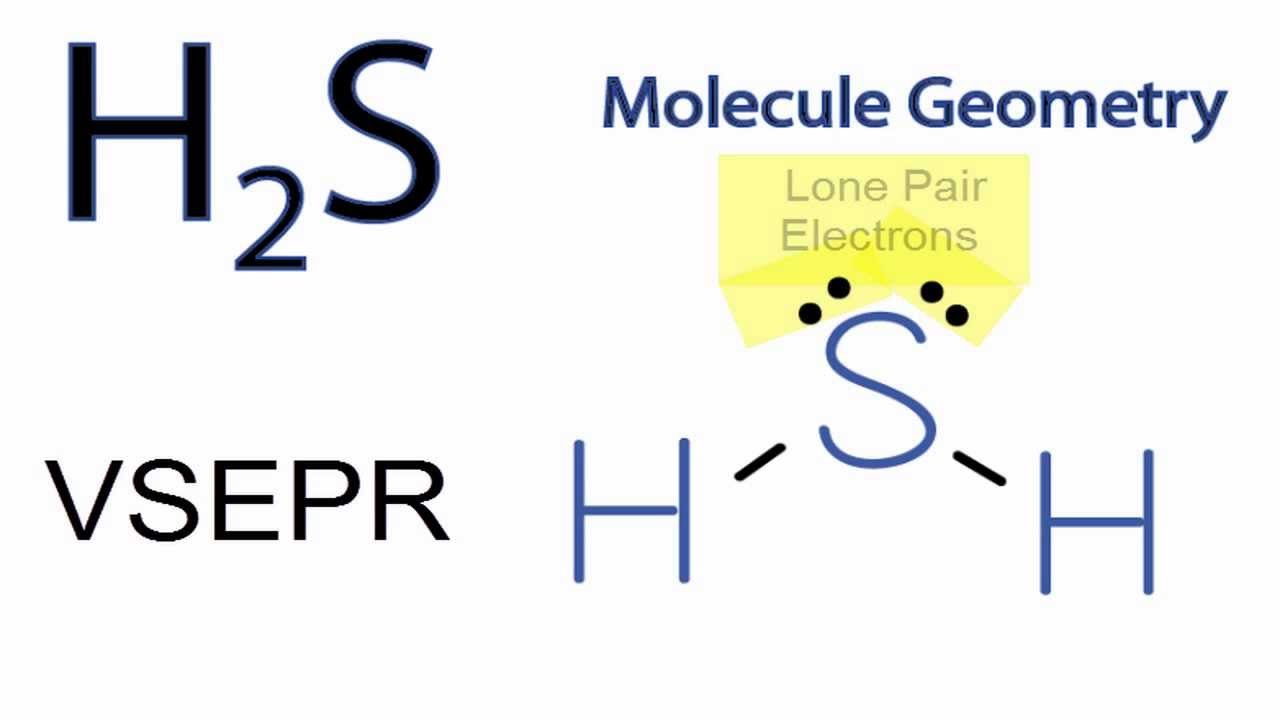
H2S Lewis Structure, Molecular Geometry, Hybridization and Polarity
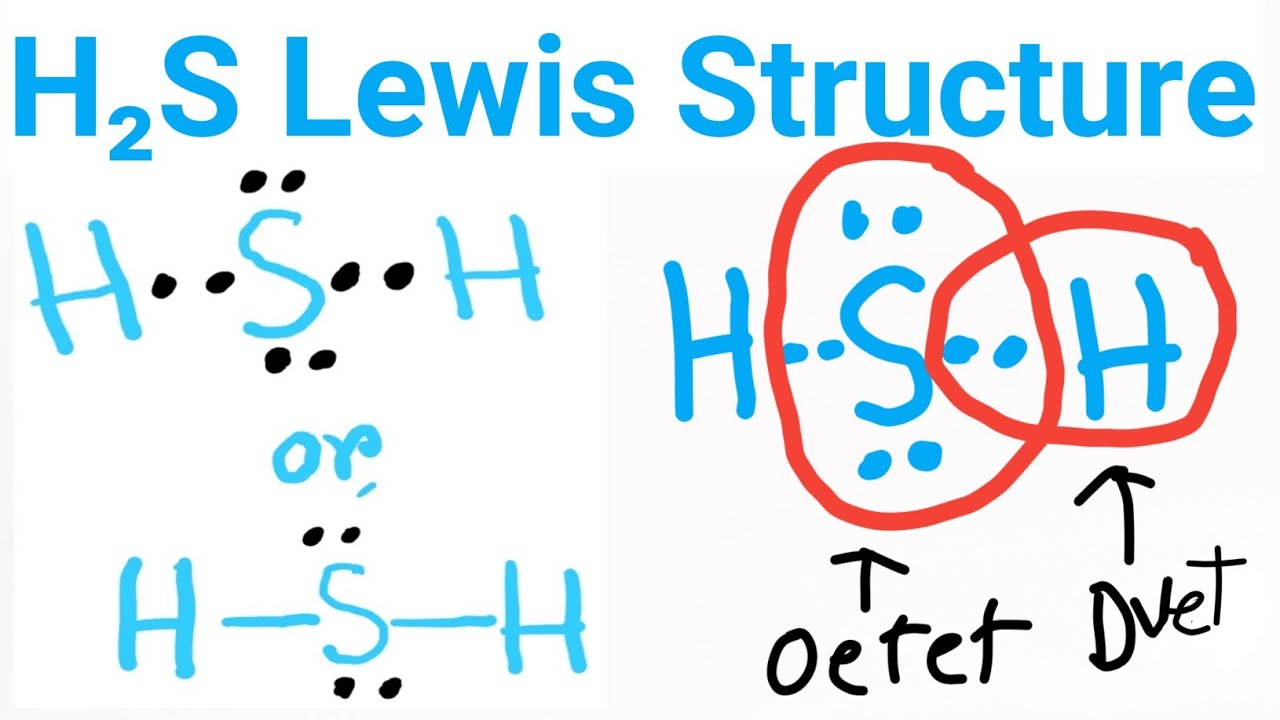
H2S Lewis Structure Lewis Dot Structure for H2S Hydrogen sulfide
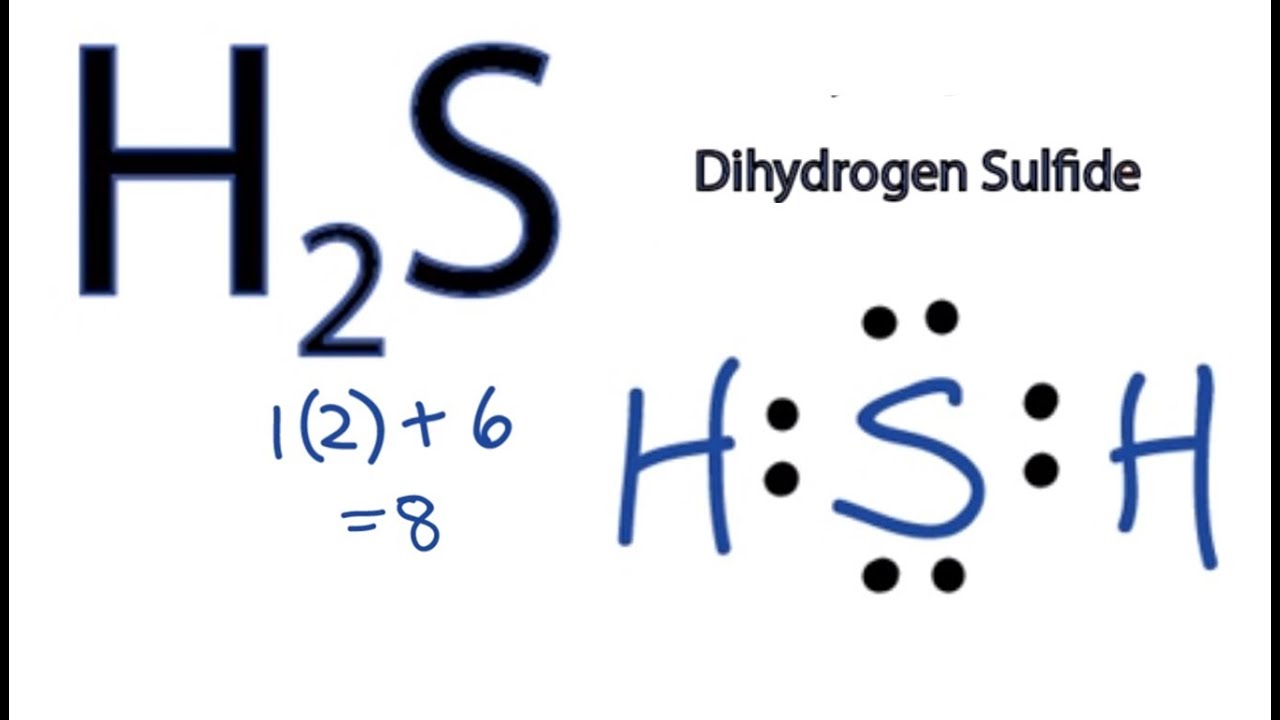
H2S Lewis Structure How to Draw the Dot Structure for H2S YouTube
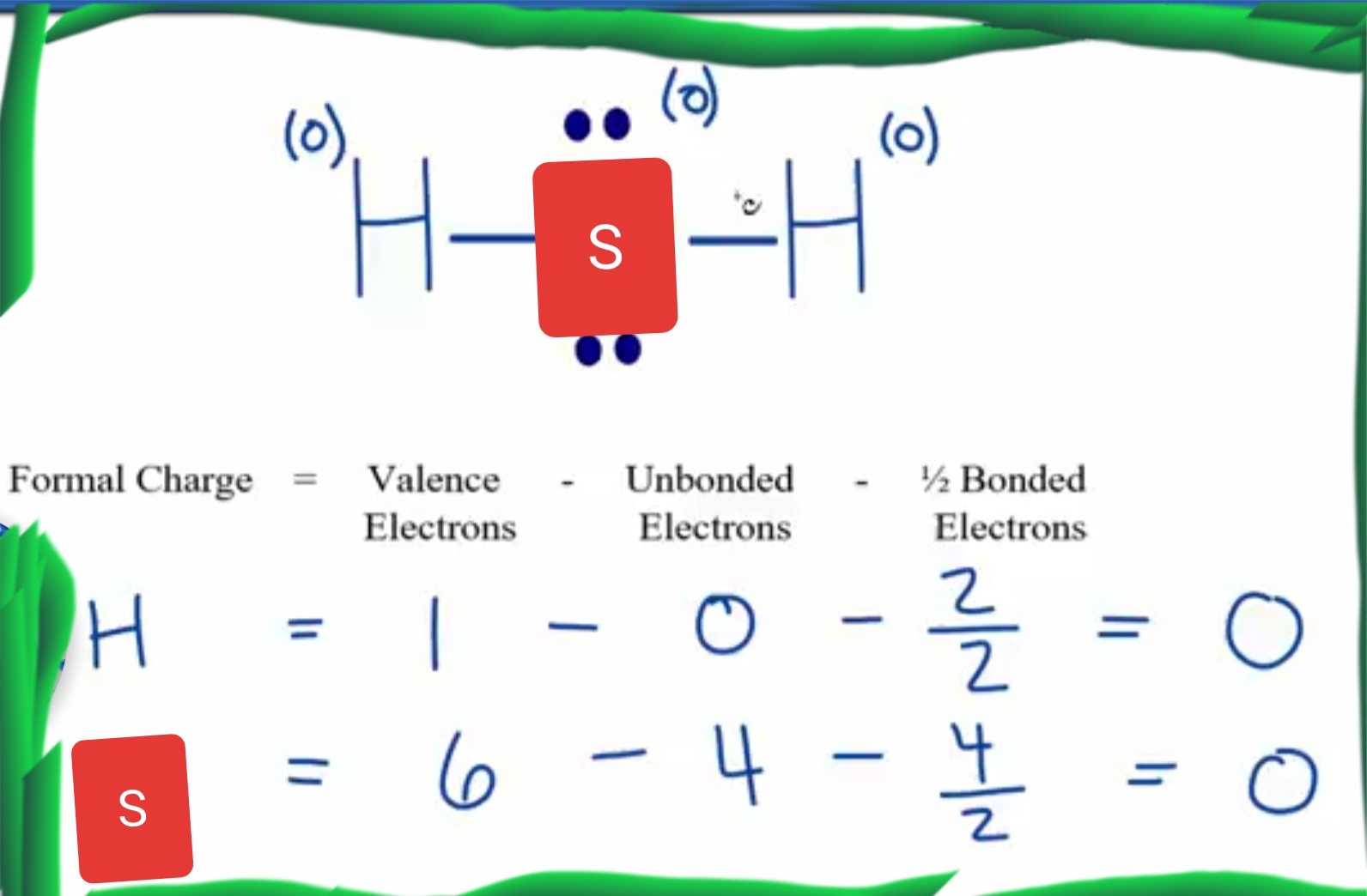
【4 Steps】H2S Lewis StructureLewis Structure for H2S (Dihydrogen
Check The Octet Rule For Each Atom.
First And Foremost It Is Important To Determine How Many Valence Electrons Are Present In The Compound.
Let’s Draw And Understand This Lewis Dot Structure Step By Step.
40K Views 2 Years Ago Lewis Structures.
Related Post: