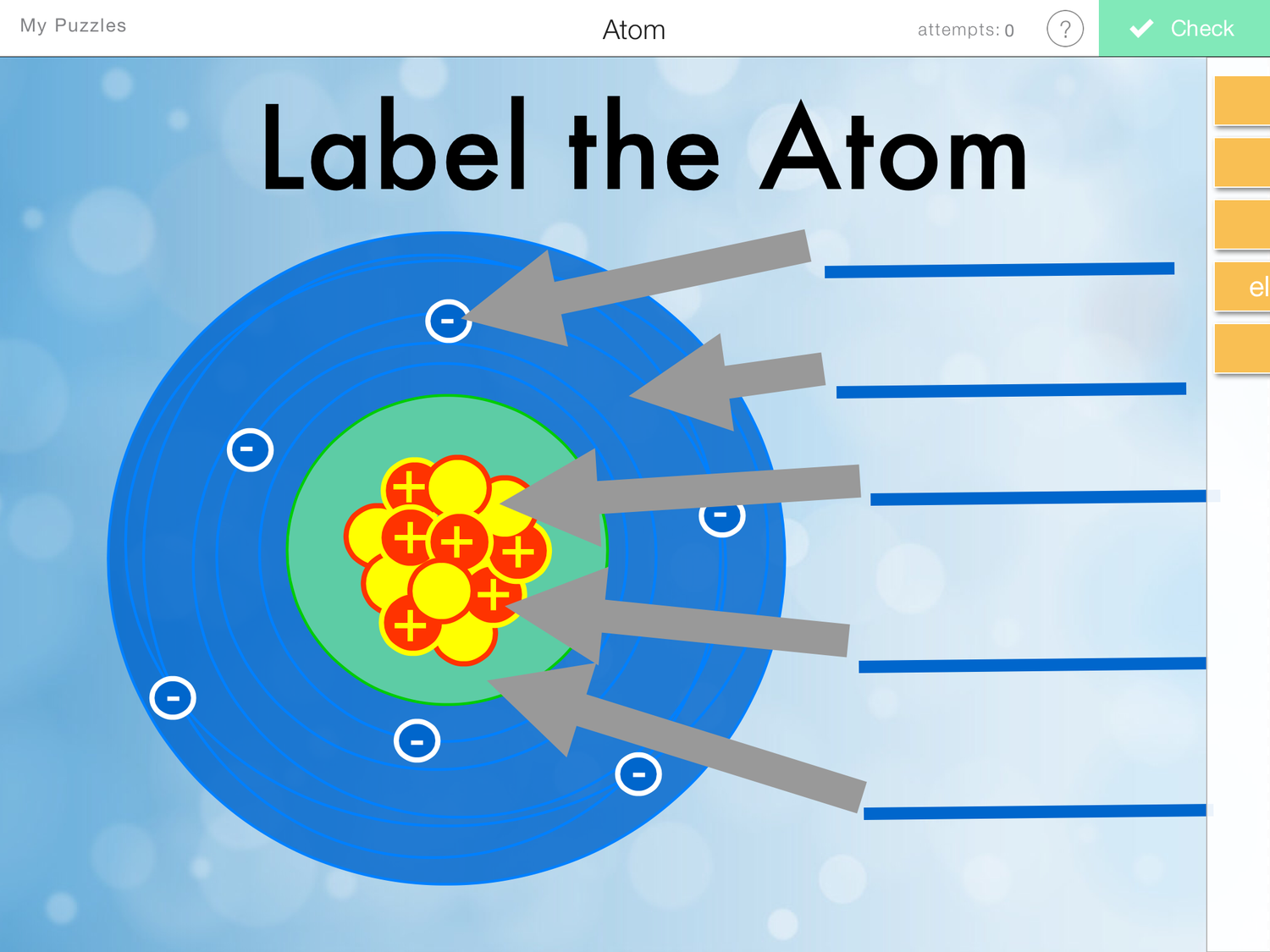
Draw Atomic Structure
Draw Atomic Structure - Web figure 2.2.1 2.2. Download complete chapter notes of structure of atom. Web the development of modern atomic theory revealed much about the inner structure of atoms. Web a layer of an atom is somewhat similar to a sheet of paper. Calculate average atomic mass and isotopic abundance. Carbon (atomic number 6) has six electrons. Web electron shells and the bohr model. The structure of the atom. Whenever an atom of an element loses one or more electrons, positive ions are formed. Web write and interpret symbols that depict the atomic number, mass number, and charge of an atom or ion. Drawing atomic structure requires only a simple understanding of the components of atomic structure. Web atomic structure and electron configuration get 3 of 4 questions to level up! Ions are those species which have a positive or a negative charge. The numbers of subatomic particles in. Web a layer of an atom is somewhat similar to a sheet of paper. Web drawing every bond and every atom is tedious, however, so chemists have devised several shorthand ways for writing structures. Drawing atomic structure requires only a simple understanding of the components of atomic structure. Level up on all the skills in this unit and collect up to 300 mastery points! The nucleus, the center of atom contain proton and neutron,. Web write and interpret symbols that depict the atomic number, mass number, and charge of an atom or ion. Ions are those species which have a positive or a negative charge. Level up on all the skills in this unit and collect up to 300 mastery points! Neutrons and protons are found at the center of the atom within a. The development of modern atomic theory revealed much about the inner structure of atoms. Web electron shells and the bohr model. If you understand how protons and electrons relate to one another, as well as how neutrons aid in comprising atomic mass, the rest is cake. It was learned that an atom contains a very small nucleus composed of positively. Web the atomic structure refers to the structure of an atom comprising a nucleus (centre) in which the protons (positively charged) and neutrons (neutral) are present. Web online chemical structure editor. Web atomic structure is the structure of an atom that consists of a nucleus (the centre), protons (positively charged), and neutrons (neutral). Following hydrogen is the noble gas helium,. Web an atom is composed of two regions: The numbers of subatomic particles in. Web online chemical structure editor. Define the atomic mass unit and average atomic mass. It is a helpful schematic to use when writing electron configurations or drawing orbital diagrams. Four of them fill the 1 s and 2 s orbitals. Calculate average atomic mass and isotopic abundance. Web drawing every bond and every atom is tedious, however, so chemists have devised several shorthand ways for writing structures. Upload a structure file or draw using a molecule editor. Web a layer of an atom is somewhat similar to a sheet. Web search by structure or substructure. Whenever an atom of an element loses one or more electrons, positive ions are formed. The negatively charged particles called electrons revolve around the centre of the nucleus. Drawchemistry lets you create structural formula online and save it as either.mol or.png. Web we recommend using the latest version of chrome, firefox, safari, or edge. Web describe the structure of an atom in terms of its protons, neutrons, and electrons. Upload a structure file or draw using a molecule editor. Define the atomic mass unit and average atomic mass. The electrons are negatively charged particles that orbit the nucleus’s centre. Atoms have protons and neutrons in the center, making the nucleus, while the electrons orbit. Level up on all the skills in this unit and collect up to 300 mastery points! Calculate average atomic mass and isotopic abundance. Four of them fill the 1 s and 2 s orbitals. Then play a game to test your ideas! Web we recommend using the latest version of chrome, firefox, safari, or edge. Web write and interpret symbols that depict the atomic number, mass number, and charge of an atom or ion. The bohr model shows the atom as a central nucleus containing protons and neutrons, with the electrons in circular electron shells at specific distances from the nucleus, similar to planets orbiting around the sun. All atoms except hydrogen contain three basic subatomic particles: Web we recommend using the latest version of chrome, firefox, safari, or edge. Atoms are the smallest units of an element. Carbon (atomic number 6) has six electrons. Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. The electrons are negatively charged particles that orbit the nucleus’s centre. Web a layer of an atom is somewhat similar to a sheet of paper. Web describe the structure of an atom in terms of its protons, neutrons, and electrons. Ions are those species which have a positive or a negative charge. Level up on all the skills in this unit and collect up to 300 mastery points! Upload a structure file or draw using a molecule editor. Quick notes structure of an atom. Define the atomic mass unit and average atomic mass. If you understand how protons and electrons relate to one another, as well as how neutrons aid in comprising atomic mass, the rest is cake.Label Parts of an Atom — Learning in Hand with Tony Vincent

Atom Definition, Structure & Parts with Labeled Diagram

How to draw Atom structure diagram step by step l Atomic structure

Simple model of atom structure with electrons vector image on
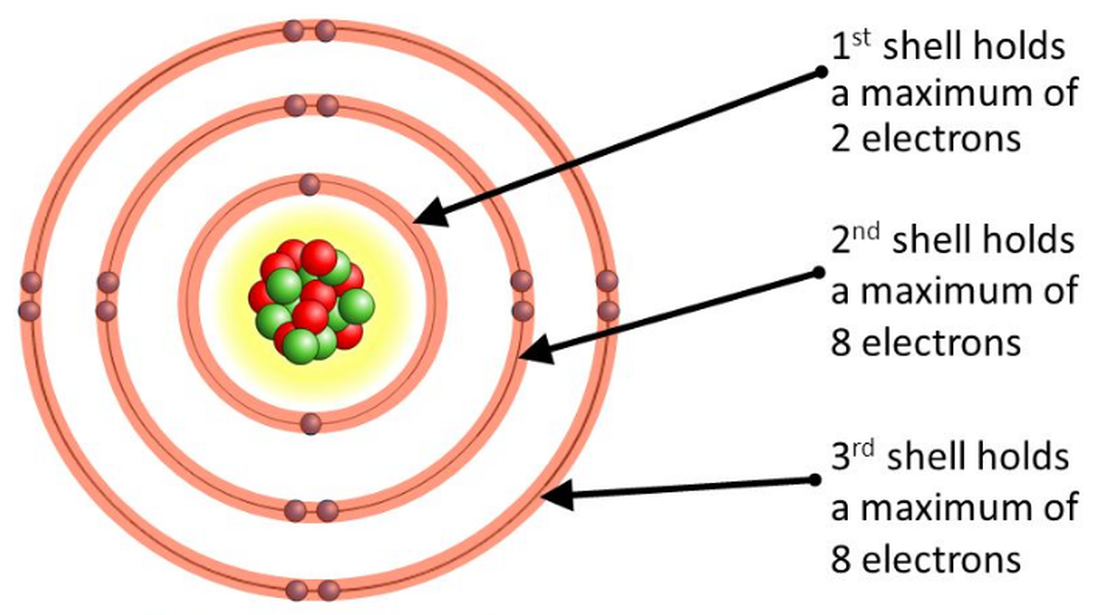
Lesson 4 THE STRUCTURE OF THE ATOM WillowWood Lessons
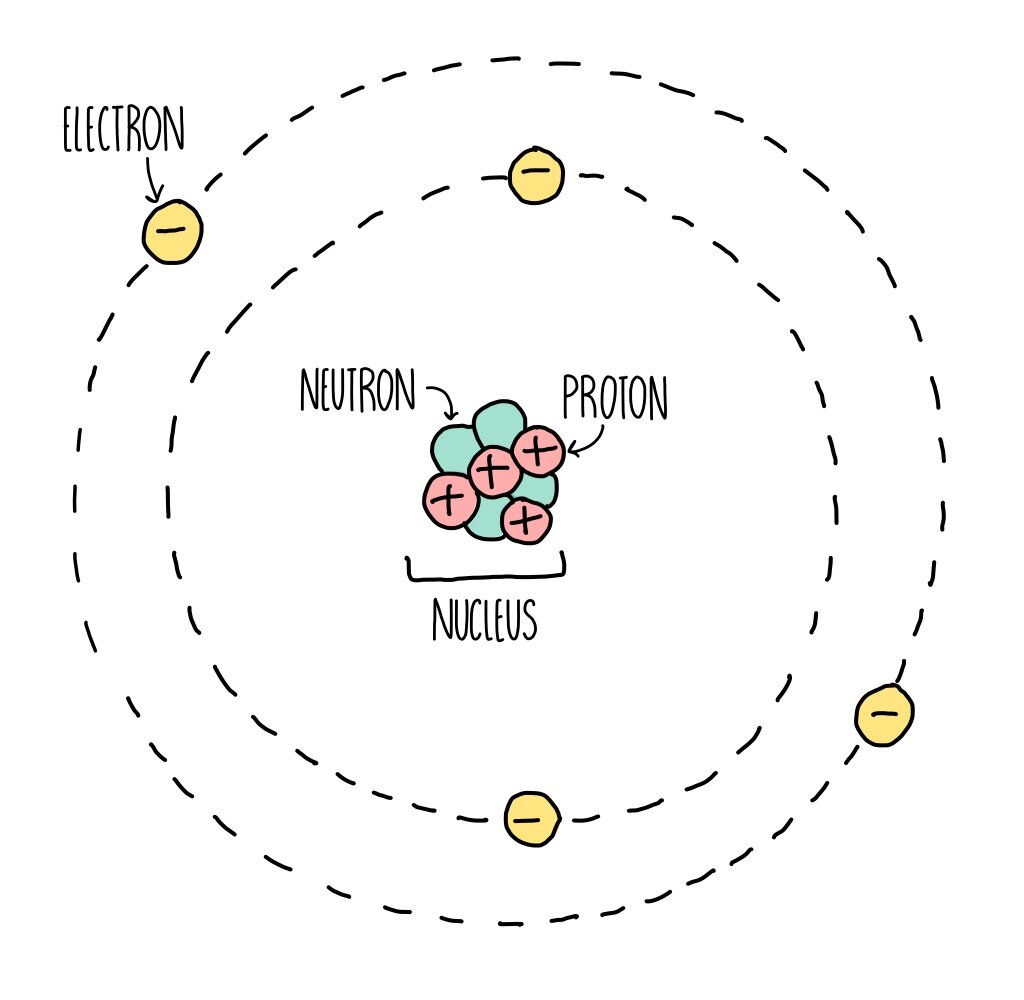
Atomic Structure (GCSE) — the science sauce

Drawing Atoms Montessori Muddle

Learn the Parts of an Atom

Atomic Structure Broad Learnings

How to Draw an Atom Really Easy Drawing Tutorial
Four Of Them Fill The 1 S And 2 S Orbitals.
Whenever An Atom Of An Element Loses One Or More Electrons, Positive Ions Are Formed.
It Is A Helpful Schematic To Use When Writing Electron Configurations Or Drawing Orbital Diagrams.
Figure 10.5C Illustrates The Traditional Way To Remember The Filling Order For Atomic Orbitals.
Related Post: