Draw And Label Water Molecule
Draw And Label Water Molecule - Web the water molecule, visualized three different ways: Because of the higher electronegativity of the oxygen atom, the bonds are polar covalent ( polar bonds ). The two hydrogen atoms as 'h'. Web because water seems so ubiquitous, many people are unaware of the unusual and unique properties of water, including: Explain what is meant by hydrogen bonding and the molecular structural features that bring it about. This diagram illustrates the unique properties of water, such as its high boiling point, surface tension, and ability to dissolve many substances. Web water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. Students will also be able to show in a drawing that the polar nature of water can explain some of water’s interesting characteristics and help explain its evaporation rate compared to a less polar liquid. Web identify three special properties of water that make it unusual for a molecule of its size, and explain how these result from hydrogen bonding. Students will also be able to show in a drawing that the polar nature of water can explain some of water’s interesting characteristics and help explain its evaporation rate compared to a less polar liquid. Students will also be able to show in a drawing that the polar nature of water can explain some of water’s interesting characteristics and help explain its evaporation rate compared to a less polar liquid. Boiling point and freezing point. These opposite charges attract each other, forming hydrogen bonds. Students will also be able to show in a drawing that. These opposite charges attract each other, forming hydrogen bonds. Web students will be able to explain, on the molecular level, what makes water a polar molecule. Web the hydrogen bond is represented by a dotted line between the molecules. Students will also be able to show in a drawing that the polar nature of water can explain some of water’s. Students will be able to explain, on the molecular level, what makes water a polar molecule. Web water is made of three atoms, an oxygen atom and two hydrogen atoms, and thus is both a molecule and a compound. Although the water as a whole is electrically neutral, it behaves as an electrical dipole. Web because water seems so ubiquitous,. Explain what is meant by hydrogen bonding and the molecular structural features that bring it about. Web strong bond within one water molecule when electrons are unequally shared. Web students will be able to explain, on the molecular level, what makes water a polar molecule. Because of the higher electronegativity of the oxygen atom, the bonds are polar covalent (. Although the water as a whole is electrically neutral, it behaves as an electrical dipole. There are two lone pairs of electrons on each oxygen atom (represented by. Label the interactions between water molecules: The oxygen atom as 'o'. Boiling point and freezing point. Web because the water molecule has an h — o — h bond angle of 105°, the molecule as a whole is polar. Students will be able to explain, on the molecular level, what makes water a polar molecule. Web water is a molecule that has a permanent dipole (i.e. Web water’s chemical formula is h 2 o. Students will. Web a water molecule consists of two hydrogen atoms bonded to an oxygen atom, and its overall structure is bent. This diagram illustrates the unique properties of water, such as its high boiling point, surface tension, and ability to dissolve many substances. Students will also be able to show in a drawing that the polar nature of water can explain. Students will also be able to show in a drawing that the polar nature of water can explain some of water’s interesting characteristics and help explain its evaporation rate compared to a less polar liquid. Web draw three water molecules, each consisting of two hydrogen (h) atoms and one oxygen (o) atom. Web water is made of three atoms, an. This is because the oxygen atom, in addition to forming bonds with the hydrogen atoms, also carries two pairs of unshared electrons. Web covers structure of water molecule, hydrogen bonding on water, polarity of water molecule, and bond angles of water molecule. There are two lone pairs of electrons on each oxygen atom (represented by. Web a water molecule consists. Web students will be able to explain, on the molecular level, what makes water a polar molecule. Web label parts of water molecule and bonds shown learn with flashcards, games, and more — for free. Because of the higher electronegativity of the oxygen atom, the bonds are polar covalent ( polar bonds ). Students will also be able to show. The oxygen atom as 'o'. Web a water molecule consists of two hydrogen atoms bonded to an oxygen atom, and its overall structure is bent. The negative charge of the electron is balanced by the positive charge of one proton in the hydrogen nucleus. The hydrogen bond between the hydrogen atom of one water molecule and the oxygen atom. Web water is a molecule that has a permanent dipole (i.e. Web molecular structure of water: These opposite charges attract each other, forming hydrogen bonds. Web water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. Web strong bond within one water molecule when electrons are unequally shared. These properties allow cells to regulate their internal temperature, provide lubrication, and facilitate nutrient uptake and waste removal. There are two lone pairs of electrons on each oxygen atom (represented by. Web covers structure of water molecule, hydrogen bonding on water, polarity of water molecule, and bond angles of water molecule. The diagram below depicts a water molecule. Oxygen has 8 protons, 8 neutrons, and 8 electrons. Use that information to draw a diagram of a water molecule that shows electron sharing between the single oxygen and the two hydrogens. Web water (h2o) should be drawn as two hydrogen atoms connected to one oxygen atom by a bond known as a polar covalent bond.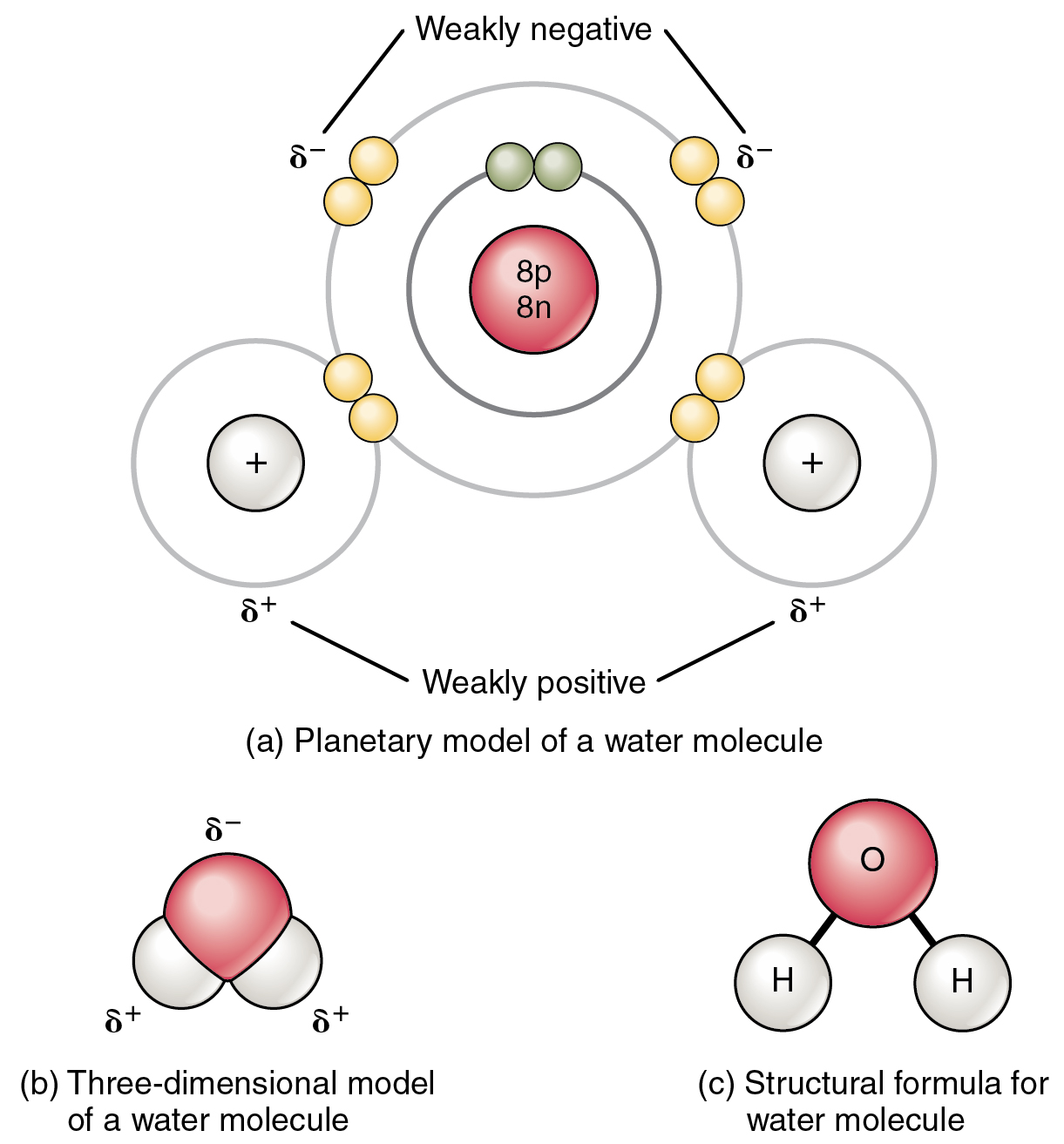
Chemical Bonds · Anatomy and Physiology

Types of Atoms Science at Your Doorstep
Diagram Of Water Molecule Labeled vrogue.co
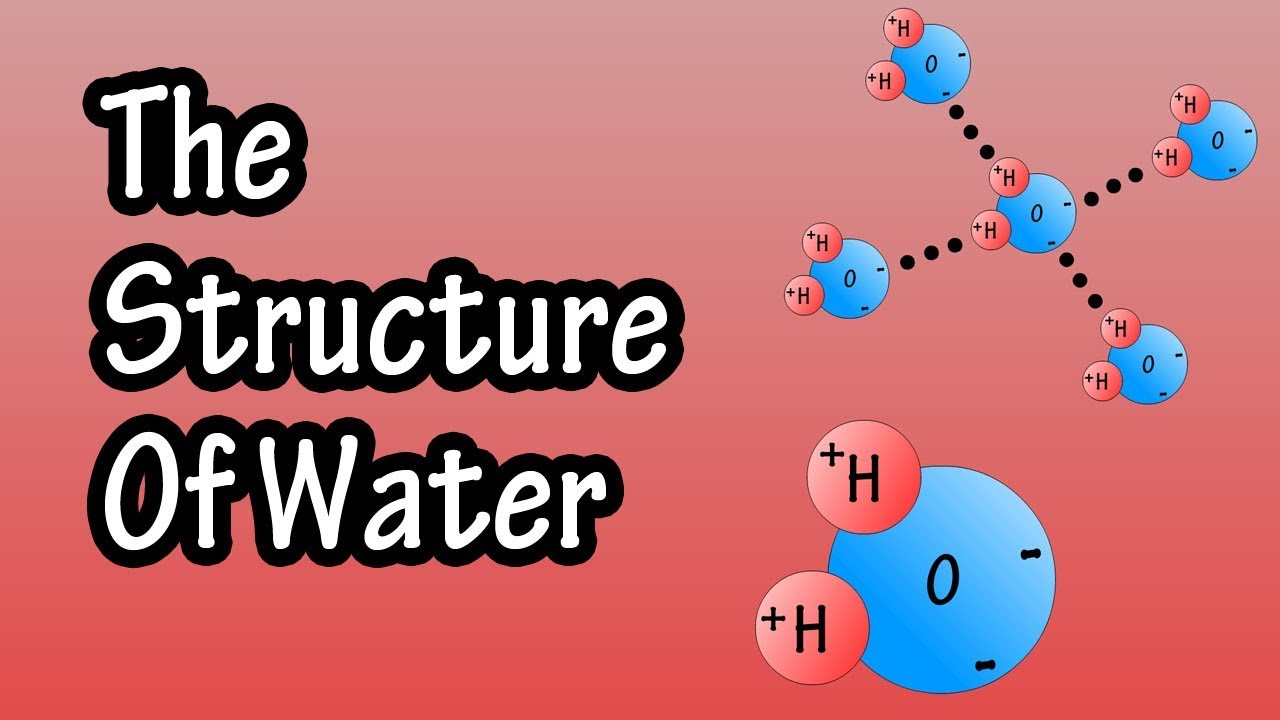
Structure Of Water Molecule Chemistry Of Water Properties Of Water
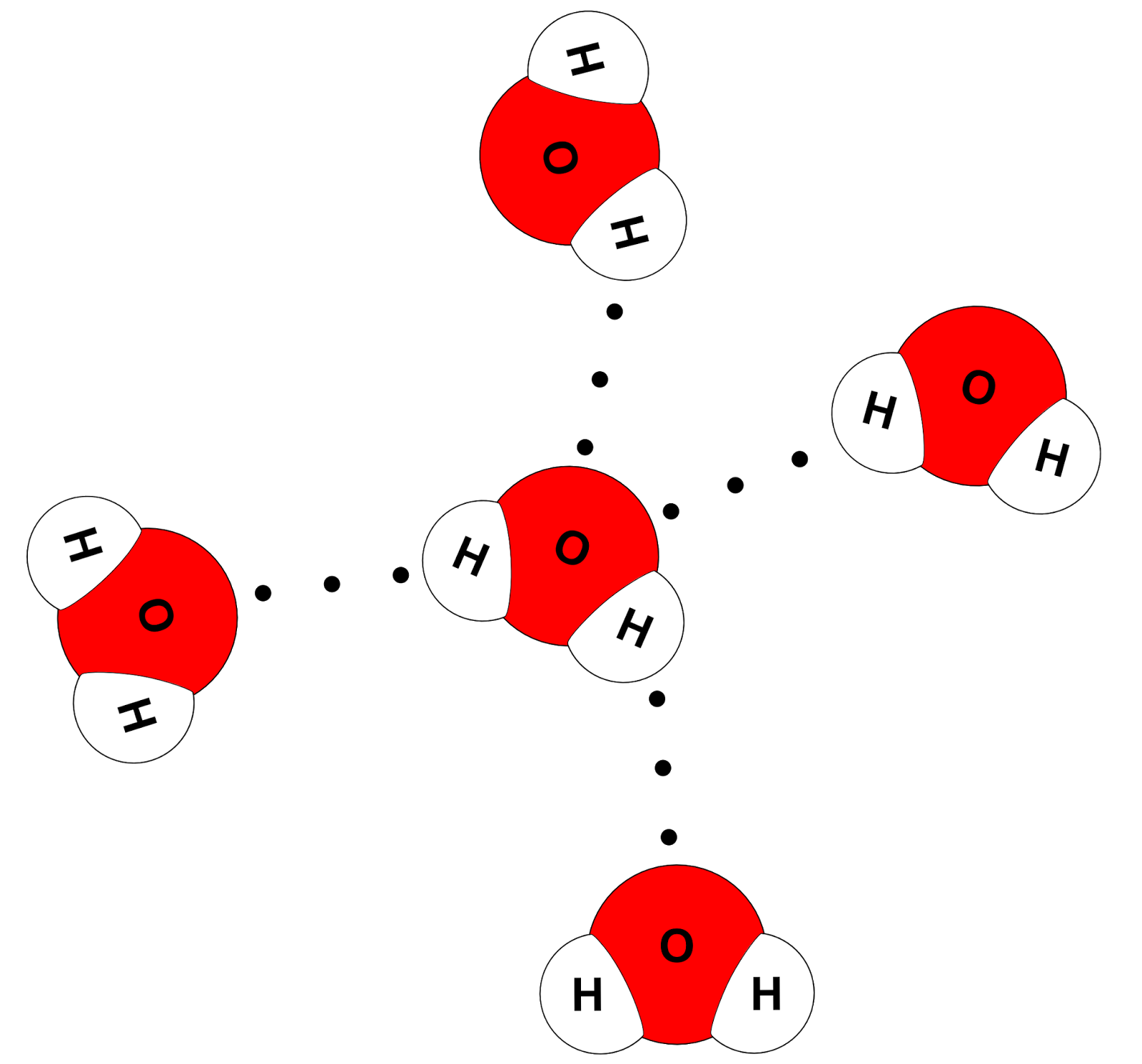
Science online The importance of the water and its structure

Water Lewis Structure How to Draw the Lewis Structure for Water YouTube
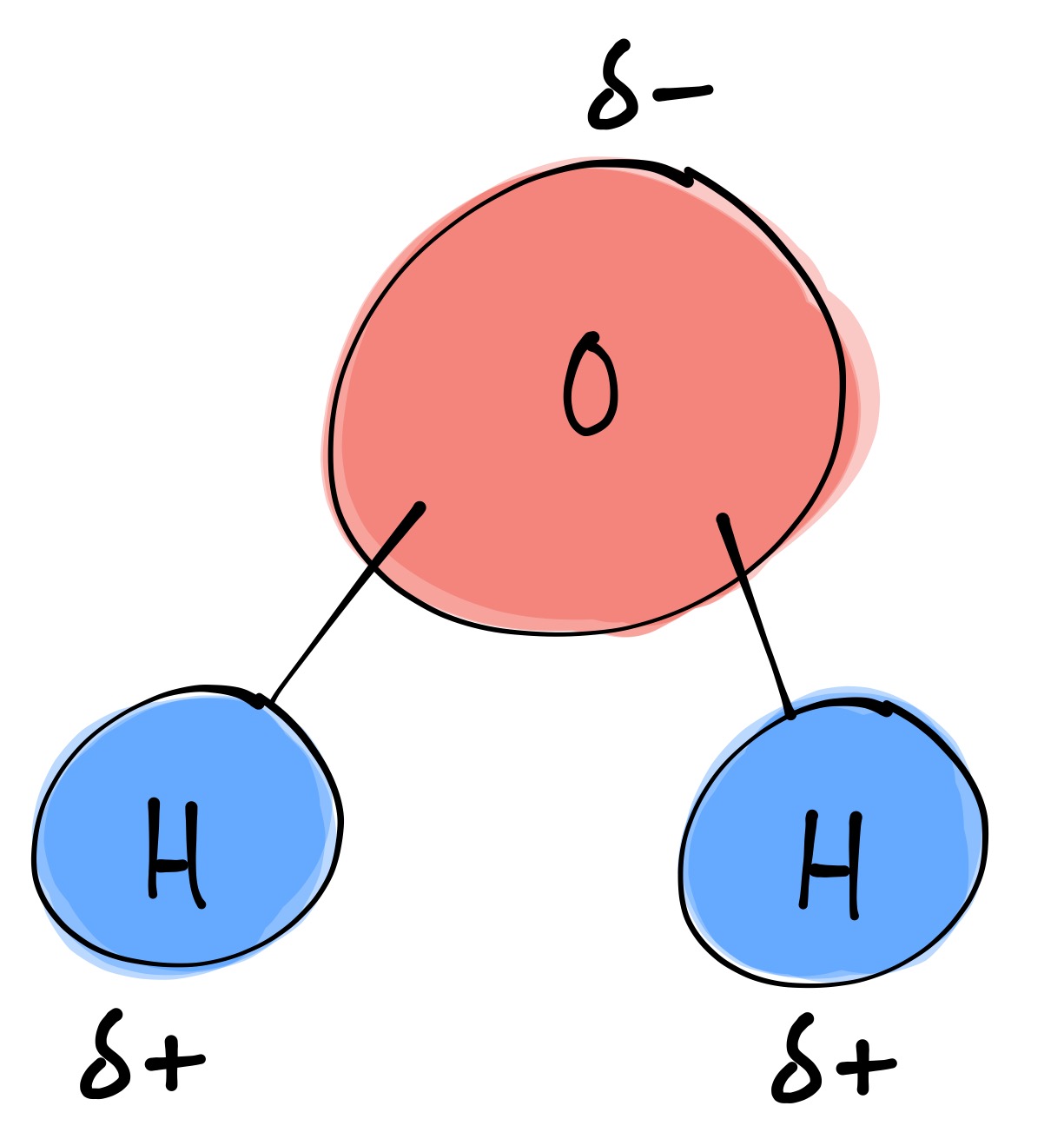
water molecule

Draw a neat well labelled diagram of information of water molecule

Water Molecule Structure For Kids

Draw a diagram of water molecules, labeling the hydrogen bond and
This Is Because The Oxygen Atom, In Addition To Forming Bonds With The Hydrogen Atoms, Also Carries Two Pairs Of Unshared Electrons.
Label The Interactions Between Water Molecules:
Surface Tension, Heat Of Vaporization, And Vapor Pressure.
It Is A Polar Molecule), With The Highly Electronegative Oxygen Nucleus Taking The Lion’s Share Of The Shared Electrons’ Time, Leaving The Hydrogen Nuclei Stripped Bare Down To Their Protons.
Related Post: