Draw 4 Water Molecules Interacting With A Li Ion
Draw 4 Water Molecules Interacting With A Li Ion - Web in contrast to an ionic solid, the structure of liquid water is not completely ordered because the interactions between molecules in a liquid are constantly breaking and reforming. Web when water molecules move closer to ions under the influence of their mutual attraction, there is a net lowering of the potential energy of the microscopic particles. Each water molecule would point its oxygen (negative dipole). And thus in aqueous solution each lithium ion is associated with, i.e. A charged or polar substance that interacts with and dissolves in water is said to be hydrophilic: Five water molecules are shown near one. This is because the positive and negative ends of water molecules are attracted to. Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. [li(oh 2)4−6]+ and [cl(h 2o)6]+. Web figure 4.1.1 the effect of charge and distance on the strength of electrostatic interactions. As the charge on ions increases or the distance between ions decreases,. Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. Web in contrast to an ionic solid, the structure of liquid water is not completely ordered because the interactions between molecules in a liquid are constantly breaking and reforming. Besides hydrogen bonds,. Five water molecules are shown near one. And thus in aqueous solution each lithium ion is associated with, i.e. Water’s solvent properties result from its polar covalent structure, allowing electrostatic interactions between water molecules at the right and nacl at the upper. This is because the positive and negative ends of water molecules are attracted to. Web where licl(aq) specifies. Web water, due to its polarity, acts as a fantastic solvent, especially for polar substances and ions. Four water molecules are shown interacting favorably with a magnesium dication. Each water molecule would point its oxygen (negative dipole). Web in contrast to an ionic solid, the structure of liquid water is not completely ordered because the interactions between molecules in a. Water molecules interact with each other through a type of interaction. Each water molecule would point its oxygen (negative dipole). Web when we add ionic compounds to water, the individual ions react with the water molecules’ polar regions and their ionic bonds are disrupted in the process of dissociation. Web water molecules are also attracted to other polar molecules and. Because of the higher electronegativity of the oxygen. Four water molecules are shown interacting favorably with a magnesium dication. Web water, due to its polarity, acts as a fantastic solvent, especially for polar substances and ions. Web when water is used as the solvent, the dissolving process is called hydration. Web water molecules are also attracted to other polar molecules. Web in contrast to an ionic solid, the structure of liquid water is not completely ordered because the interactions between molecules in a liquid are constantly breaking and reforming. As the charge on ions increases or the distance between ions decreases,. Water molecules interact with each other through a type of interaction. A charged or polar substance that interacts with. Web where licl(aq) specifies the aquated ions, i.e. Water molecules interact with each other through a type of interaction. Four water molecules are shown interacting favorably with a magnesium dication. This is because the positive and negative ends of water molecules are attracted to. Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. As the charge on ions increases or the distance between ions decreases,. Five water molecules are shown near one. Web a water molecule is made up of two hydrogen atoms connected by covalent bonds to one oxygen atom. Web when we add ionic compounds to water, the individual ions react with the water molecules’ polar regions and their ionic bonds. Web when water molecules move closer to ions under the influence of their mutual attraction, there is a net lowering of the potential energy of the microscopic particles. Web when we add ionic compounds to water, the individual ions react with the water molecules’ polar regions and their ionic bonds are disrupted in the process of dissociation. Because of the. The negative ends of the water dipoles are directed. Web water molecules are also attracted to other polar molecules and to ions. Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. Web water, due to its polarity, acts as a fantastic solvent, especially for polar substances and ions. Web in contrast to an. Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. Web when water is used as the solvent, the dissolving process is called hydration. Web when we add ionic compounds to water, the individual ions react with the water molecules’ polar regions and their ionic bonds are disrupted in the process of dissociation. Because of the higher electronegativity of the oxygen. Besides hydrogen bonds, what other intermolecular forces could be possible between a water molecule and an ethanol. Water molecules interact with each other through a type of interaction. Web water molecules are also attracted to other polar molecules and to ions. Four water molecules are shown interacting favorably with a magnesium dication. A charged or polar substance that interacts with and dissolves in water is said to be hydrophilic: The negative ends of the water dipoles are directed. Five water molecules are shown near one. And thus in aqueous solution each lithium ion is associated with, i.e. Web in contrast to an ionic solid, the structure of liquid water is not completely ordered because the interactions between molecules in a liquid are constantly breaking and reforming. Web where licl(aq) specifies the aquated ions, i.e. Water’s solvent properties result from its polar covalent structure, allowing electrostatic interactions between water molecules at the right and nacl at the upper. What interactions occur between two hexane molecules?
The Structure and Properties of Water / Introduction to Chemistry
[Solved] Draw a spacefilling model of 4 water molecules hydrogen
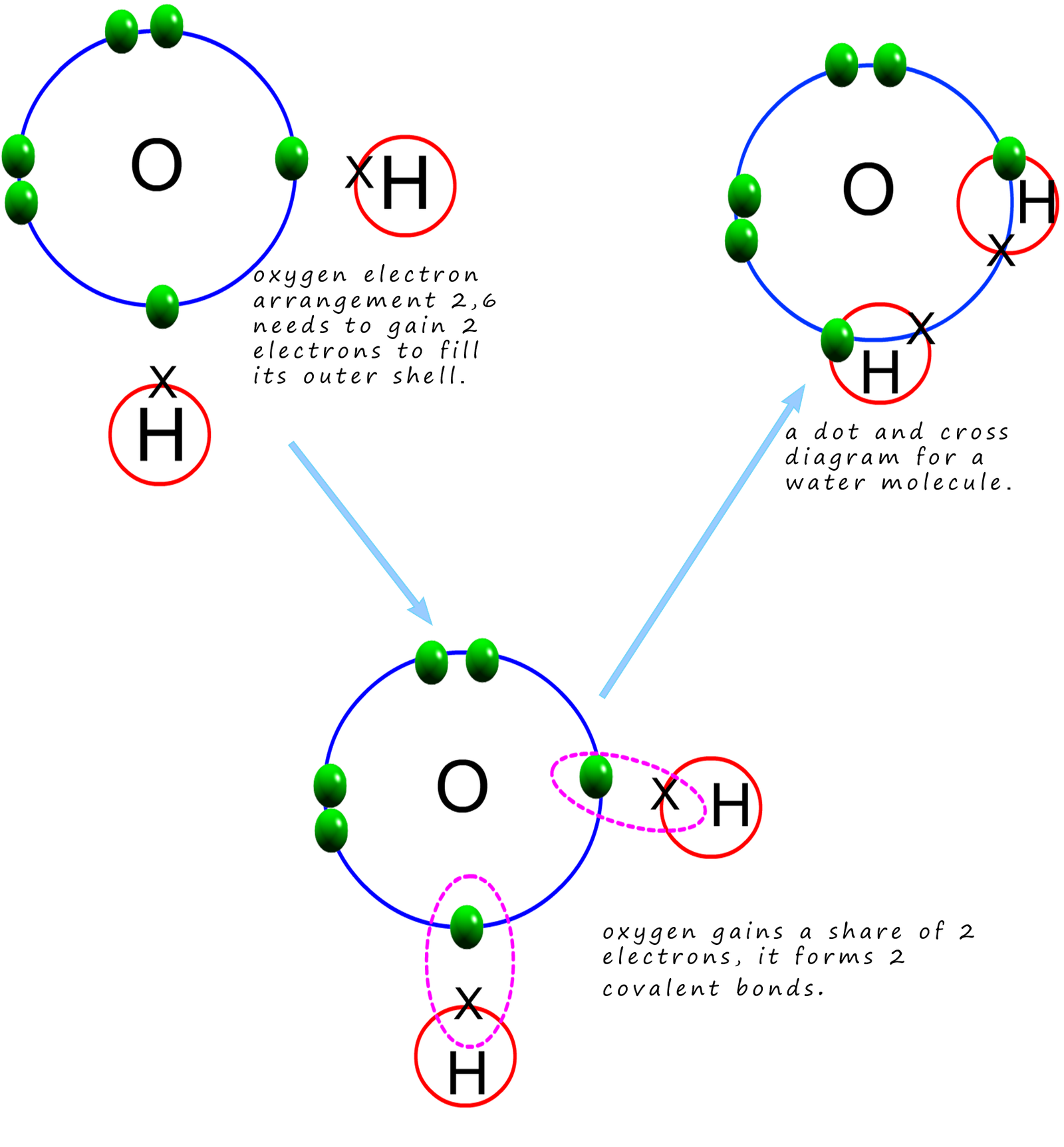
Covalent bonding
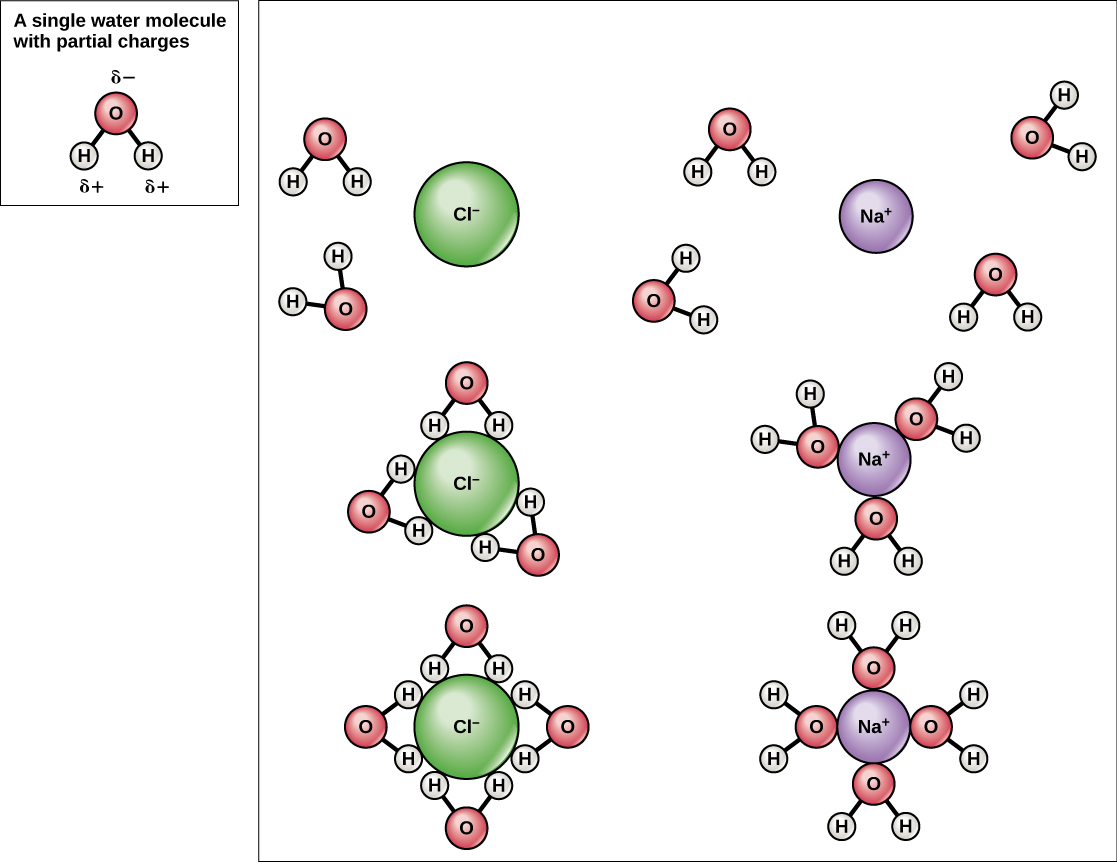
2.4 Water A Vital Compound Biology LibreTexts

3.1.4 Draw and label a diagram showing the structure of water molecules

Schematic diagram showing interaction of water molecules with
Solved Draw 4 water molecules interacting with Li+ion. (6

Intermolecular Forces Chemistry

Types of Atoms Science at Your Doorstep

Draw A Diagram Of Water Molecules Labeling The Hydrogen Bond And
Web Figure 4.1.1 The Effect Of Charge And Distance On The Strength Of Electrostatic Interactions.
Web When Water Molecules Move Closer To Ions Under The Influence Of Their Mutual Attraction, There Is A Net Lowering Of The Potential Energy Of The Microscopic Particles.
This Is Because The Positive And Negative Ends Of Water Molecules Are Attracted To.
The Interaction Between Water Molecules And Sodium Ion Is Illustrated As One Of The Diagram.
Related Post:
