Dissimilar Metals Corrosion Chart
Dissimilar Metals Corrosion Chart - Web galvanic corrosion is an electrochemical process in which one metal corrodes preferentially to another when both metals are in electrical contact, in the. Web the following three factors often contribute to a higher risk of galvanic corrosion: If any of these factors is absent, galvanic corrosion cannot occur. Web however, you can completely avoid galvanic corrosion by choosing matching metal anchors. Galvanic corrosion happens when dissimilar metals rub against one. Galvanic corrosion (also called bimetallic corrosion or dissimilar metal corrosion) is an electrochemical process in which one metal corrodes preferentially when it is in electrical contact with another, in the presence of an electrolyte. Web below is a galvanic reaction chart for dissimilar metals. Contact a corrosion specialist to determine the best. When dissimilar metals are used together in the presence of an electrolyte,. Web the common factors are dissimilar metals, electrical contact, and a conductive electrolyte in contact with them. Obviously galvanic corrosion is more. Galvanic corrosion happens when dissimilar metals rub against one. When dissimilar metals are used together in the presence of an electrolyte,. Web conductive path connects the two metals. Web the common factors are dissimilar metals, electrical contact, and a conductive electrolyte in contact with them. Web the following three factors often contribute to a higher risk of galvanic corrosion: Dissimilar metals used in conjunction; A typical rule of thumb is that voltage differences. Metals close to one another on the chart. Web galvanic corrosion potential is a measure of how dissimilar metals will corrode when placed against each other in an assembly. Web galvanic corrosion potential is a measure of how dissimilar metals will corrode when placed against each other in an assembly. Web the most important type of corrosion for understanding dissimilar metals is galvanic corrosion. Web galvanic/dissimilar metal or bimetallic corrosion is a type of electrochemical corrosion, where a material corrodes if it comes in contact with another material in. This chart is designed to assist in broadly assessing the risk of galvanic corrosion associated with a given metal coming. Web the galvanic series chart below shows metals and their electrochemical voltage range (relative activity in flowing sea water). Web this slide includes a chart of galvanic corrosion potential between common construction metals. Web galvanic corrosion is an electrochemical process. Web galvanic corrosion potential is a measure of how dissimilar metals will corrode when placed against each other in an assembly. In the case of galvanic corrosion, the combination of two dissimilar metals with an electrolyte is all that is needed to form a. Contact a corrosion specialist to determine the best. Web galvanic corrosion (also called ' dissimilar metal. In the case of galvanic corrosion, the combination of two dissimilar metals with an electrolyte is all that is needed to form a. Web galvanic corrosion potential is a measure of how dissimilar metals will corrode when placed against each other in an assembly. Web metals are rated in their ability to resist electrochemical corrosion on the scale of nobility. Web galvanic corrosion (also called ' dissimilar metal corrosion' or wrongly 'electrolysis') refers to corrosion damage induced when two dissimilar materials are coupled in a corrosive. Web the following three factors often contribute to a higher risk of galvanic corrosion: Web the most important type of corrosion for understanding dissimilar metals is galvanic corrosion. In the case of galvanic corrosion,. Web the most important type of corrosion for understanding dissimilar metals is galvanic corrosion. If any of these factors is absent, galvanic corrosion cannot occur. Galvanic corrosion happens when dissimilar metals rub against one. Web the common factors are dissimilar metals, electrical contact, and a conductive electrolyte in contact with them. Galvanic corrosion (also called bimetallic corrosion or dissimilar metal. So, for example, choosing zinc on zinc would have the lowest. A typical rule of thumb is that voltage differences. Obviously galvanic corrosion is more. Web metals listed on the top of the chart (anodic) will corrode faster than the metals on the bottom of the chart (cathodic). Web galvanic/dissimilar metal or bimetallic corrosion is a type of electrochemical corrosion,. A typical rule of thumb is that voltage differences. Web galvanic corrosion (also called ' dissimilar metal corrosion' or wrongly 'electrolysis') refers to corrosion damage induced when two dissimilar materials are coupled in a corrosive. Web below is a galvanic reaction chart for dissimilar metals. Web galvanic corrosion potential is a measure of how dissimilar metals will corrode when placed. A similar galvanic reaction is exploited in primary cells to generate a useful electrical voltage to power portable devices. This chart is designed to assist in broadly assessing the risk of galvanic corrosion associated with a given metal coming. Web below is a galvanic reaction chart for dissimilar metals. This phenomenon is named after italian ph… Metals close to one another on the chart. Contact a corrosion specialist to determine the best. A typical rule of thumb is that voltage differences. Web the larger the separation distance in the electromotive chart between the two metals in contact, the higher the contact potential and chances for corrosion. Web galvanic corrosion potential is a measure of how dissimilar metals will corrode when placed against each other in an assembly. Web the following three factors often contribute to a higher risk of galvanic corrosion: In the case of galvanic corrosion, the combination of two dissimilar metals with an electrolyte is all that is needed to form a. Web galvanic corrosion (also called ' dissimilar metal corrosion' or wrongly 'electrolysis') refers to corrosion damage induced when two dissimilar materials are coupled in a corrosive. Obviously galvanic corrosion is more. Web galvanic corrosion (some times called dissimilar metal corrosion) is the process by which the materials in contact with each other oxidizes or corrodes. Web conductive path connects the two metals. Web galvanic corrosion may be experienced when two dissimilar metals or alloys, not in direct contact, are nevertheless connected electrically.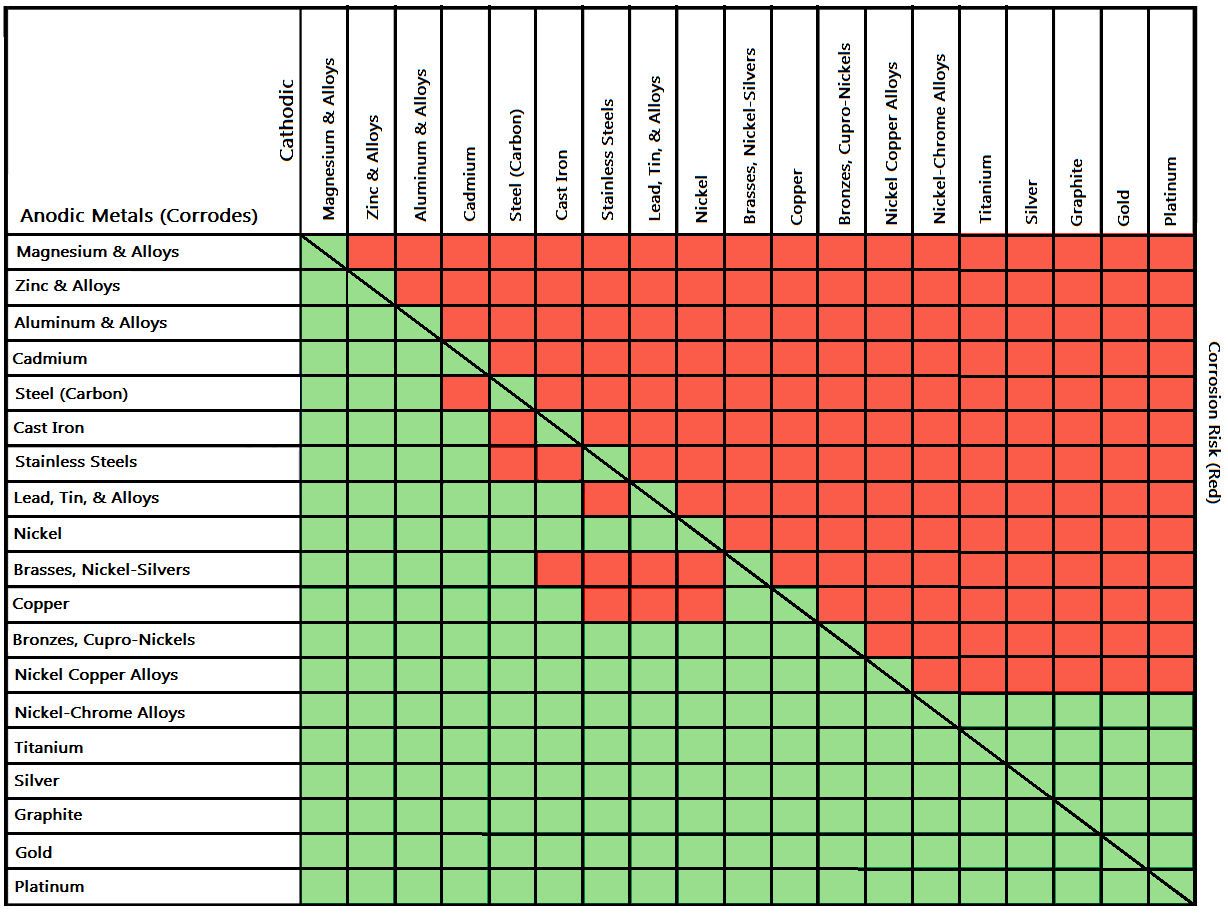
Galvanic Corrosion Common Questions Answered

21 Lovely Galvanic Corrosion Chart Dissimilar Metals Chart Gallery

Metals Galvanic Compatibility Chart A Visual Reference of Charts

FAQ 1 Galvanic/Dissimilar Metal Corrosion

Protection of Dissimilar Metal Contacts and Corrosion Limits

Stainless Steel Galvanic Corrosion Chart
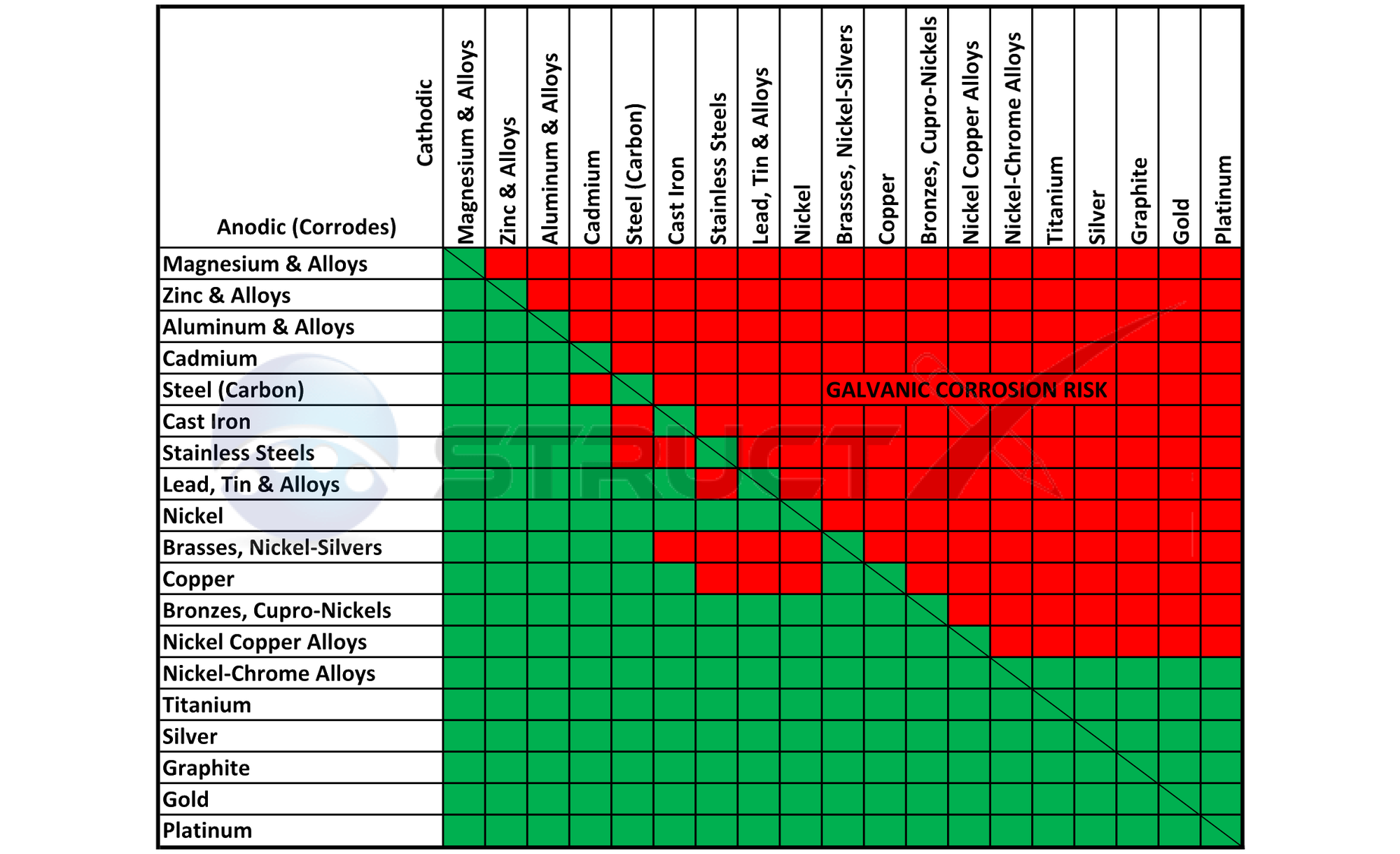
Aluminum Corrosion Resistance Chart
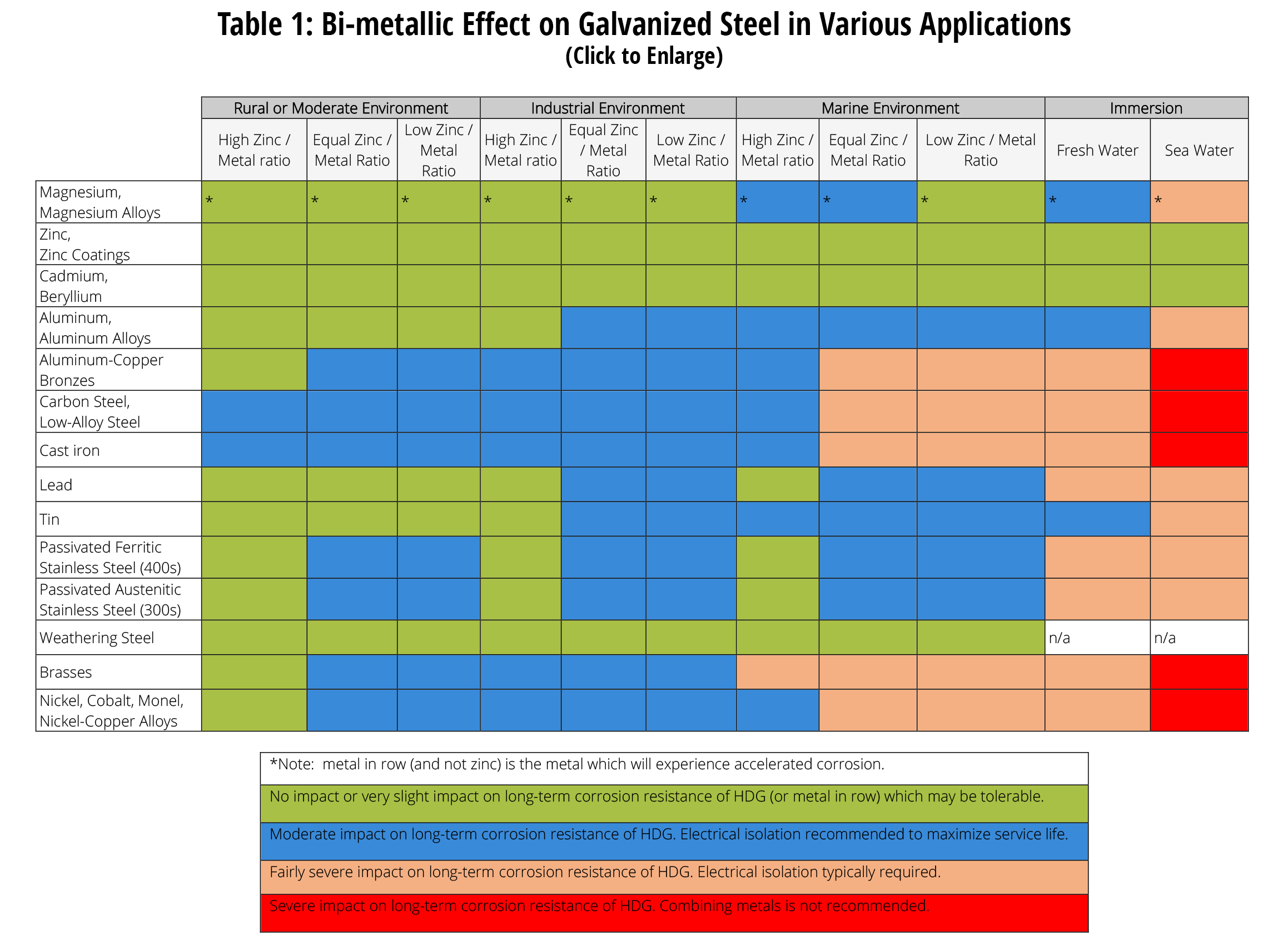
Dissimilar Metal Corrosion with… American Galvanizers Association

Galvanic Corrosion Chart Dissimilar Metals Video Bokep Ngentot
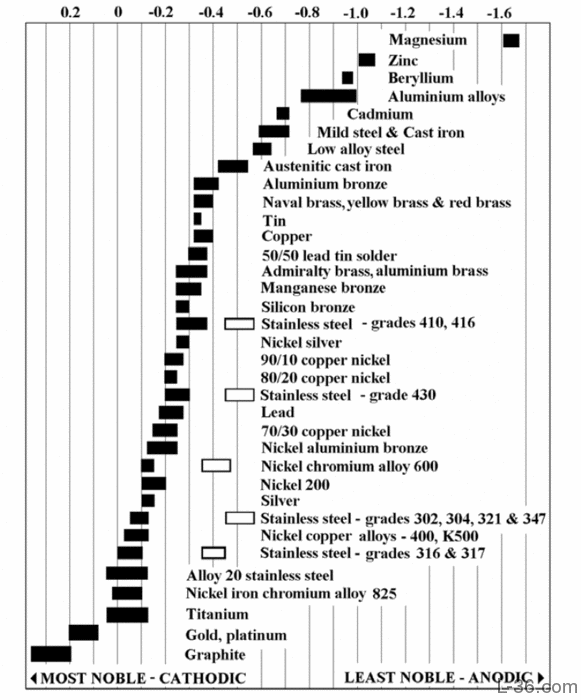
Dissimilar Corrosion Materials Tables
Galvanic Corrosion (Also Called Bimetallic Corrosion Or Dissimilar Metal Corrosion) Is An Electrochemical Process In Which One Metal Corrodes Preferentially When It Is In Electrical Contact With Another, In The Presence Of An Electrolyte.
Web The Most Important Type Of Corrosion For Understanding Dissimilar Metals Is Galvanic Corrosion.
So, For Example, Choosing Zinc On Zinc Would Have The Lowest.
When Dissimilar Metals Are Used Together In The Presence Of An Electrolyte,.
Related Post: