Clinimix Compatibility Chart
Clinimix Compatibility Chart - Web clinimix n17g35e, solution for infusion 2. Web infusion rate chart • clinimix and clinimix e solutions containing more than 5% dextrose have an osmolarity of ≥ 900 mosm/l and must be infused through a central. Web although clinimix e products contains electrolytes, supplementations may be indicated according to the clinical needs of the patient. 8% amino acids (blend d). Web the most discussed change in parenteral nutrition compatibility within the last two years was with ceftriaxone. Compatibility of the additives with the. Clinimix e 2.75/10, 4.25/10, 4.25/25, 5/15, 5/20, 5/25 o for central or peripheral vein infusion: Web 5% amino acids (blend c) without electrolytes in 16.6% dextrose injection. If required, additional amino acids may be. Web the clinimix e dosage can be adjusted based on the severity of kidney disease, supplementing protein as indicated. Qualitative and quantitative composition clinimix [n9g15e] [n9g20e] [n12g20] [n12g20e] [n14g30]. Compatibility of the additives with the product must be. Web electrolyte supplementation for clinimix products may be indicated according to the clinical needs of the patient. Web monitor severely undernourished patients and slowly increase nutrient intakes. 8% amino acids (blend d). 6% amino acids (blend d) without electrolytes in 5% dextrose injection. If required, additional amino acids may be. Clinimix e 2.75/10, 4.25/10, 4.25/25, 5/15, 5/20, 5/25 o for central or peripheral vein infusion: Web electrolyte supplementation for clinimix products may be indicated according to the clinical needs of the patient. Web clinimix n17g35e, solution for infusion 2. Web although clinimix e products contains electrolytes, supplementations may be indicated according to the clinical needs of the patient. Web 1 indications and usage. Clinimix and clinimix e solutions containing more than 5% dextrose have an osmolarity of ≥. Compatibility of the additives with the product must be. In the summer of 2007, roche laboratories updated their. Web 5% amino acids (blend c) without electrolytes in 16.6% dextrose injection. If required, additional amino acids may be added to. If required, additional amino acids may be. 8% amino acids (blend d). Web clinimix (amino acids in dextrose) injections and clinimix e (amino acids with electrolytes in dextrose with calcium) injections are indicated as a source of calories and. Web electrolyte supplementation for clinimix products may be indicated according to the clinical needs of the patient. Clinimix and clinimix e solutions containing more than 5% dextrose have an osmolarity of ≥. Web clinimix n17g35e, solution for infusion 2. If required, additional amino acids may be added to. Web the most discussed change in parenteral nutrition compatibility within the last. Web clinimix n17g35e, solution for infusion 2. If required, additional amino acids may be added to. Web this document is aimed at dispelling common myths related to patient conditions, pn components, stability and compatibility, and administration. Web advancing clinical nutrition | clinical nutrition emea If required, additional amino acids may be. Clinimix and clinimix e solutions containing more than 5% dextrose have an osmolarity of ≥. Web clinimix (amino acids in dextrose) injections and clinimix e (amino acids with electrolytes in dextrose with calcium) injections are indicated as a source of calories and. Web o for central vein infusion only: Web the most discussed change in parenteral nutrition compatibility within the. Clinimix e 2.75/5 and 4.25/5 • the solution should. Compatibility of the additives with the product must be. If required, additional amino acids may be added to. Web monitor severely undernourished patients and slowly increase nutrient intakes. Qualitative and quantitative composition clinimix [n9g15e] [n9g20e] [n12g20] [n12g20e] [n14g30]. Clinimix e 2.75/5 and 4.25/5 • the solution should. Drugs must not be added to pn bags. Web this document is aimed at dispelling common myths related to patient conditions, pn components, stability and compatibility, and administration. Clinimix e is indicated as a source of calories, protein, and electrolytes for patients requiring parenteral nutrition when oral or enteral nutrition is. Clinimix e 2.75/10, 4.25/10, 4.25/25, 5/15, 5/20, 5/25 o for central or peripheral vein infusion: Web this document is aimed at dispelling common myths related to patient conditions, pn components, stability and compatibility, and administration. Web the clinimix e dosage can be adjusted based on the severity of kidney disease, supplementing protein as indicated. Web infusion rate chart • clinimix. Web monitor severely undernourished patients and slowly increase nutrient intakes. In the summer of 2007, roche laboratories updated their. Web this document is aimed at dispelling common myths related to patient conditions, pn components, stability and compatibility, and administration. Web advancing clinical nutrition | clinical nutrition emea Web the clinimix dosage can be adjusted based on the severity of kidney disease, supplementing protein as indicated. Web 5% amino acids (blend c) without electrolytes in 16.6% dextrose injection. If required, additional amino acids may be added to. Web the most discussed change in parenteral nutrition compatibility within the last two years was with ceftriaxone. Web 1 indications and usage. If required, additional amino acids may be. Web electrolyte supplementation for clinimix products may be indicated according to the clinical needs of the patient. Clinimix and clinimix e solutions containing more than 5% dextrose have an osmolarity of ≥. Clinimix e is indicated as a source of calories, protein, and electrolytes for patients requiring parenteral nutrition when oral or enteral nutrition is not. Clinimix e 2.75/10, 4.25/10, 4.25/25, 5/15, 5/20, 5/25 o for central or peripheral vein infusion: Web we strove to evaluate and present the available published data as a comprehensive and practical reference. Drugs must not be added to pn bags.
Clinimix FDA prescribing information, side effects and uses

Iv Drug Compatibility Chart amulette

Clinimix E FDA prescribing information, side effects and uses

Table 1 from Compatibility of Lactated Ringer’s Injection With 94

Clinimix FDA prescribing information, side effects and uses

Clinimix FDA prescribing information, side effects and uses

Clinimix FDA prescribing information, side effects and uses
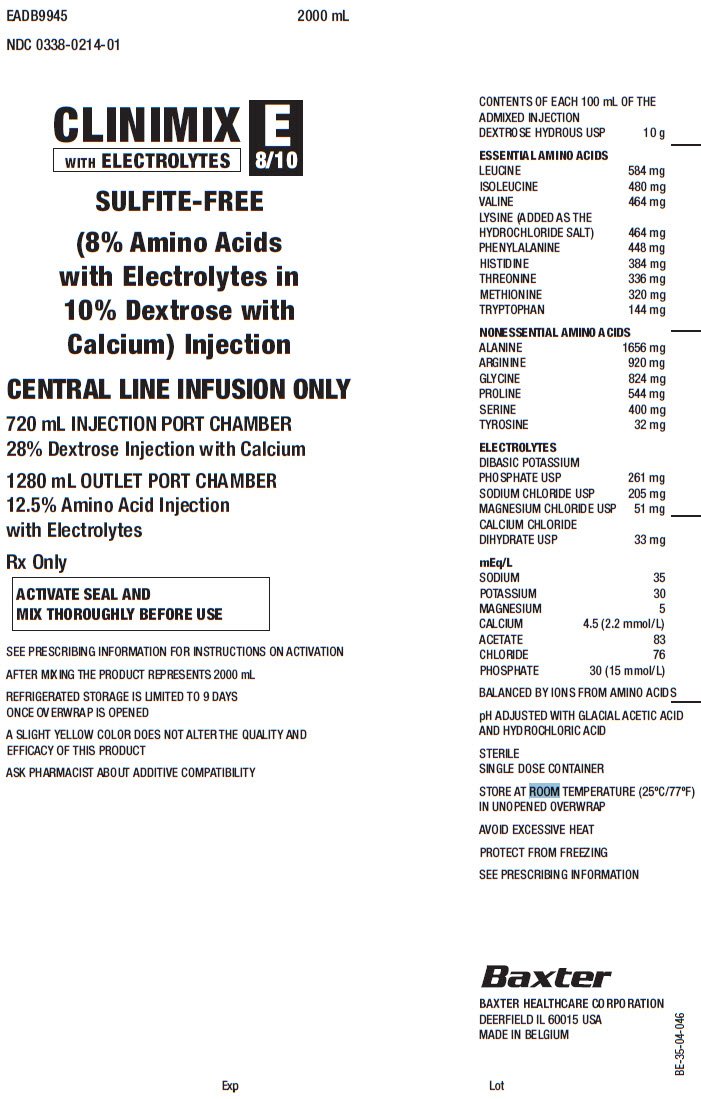
Clinimix E FDA prescribing information, side effects and uses
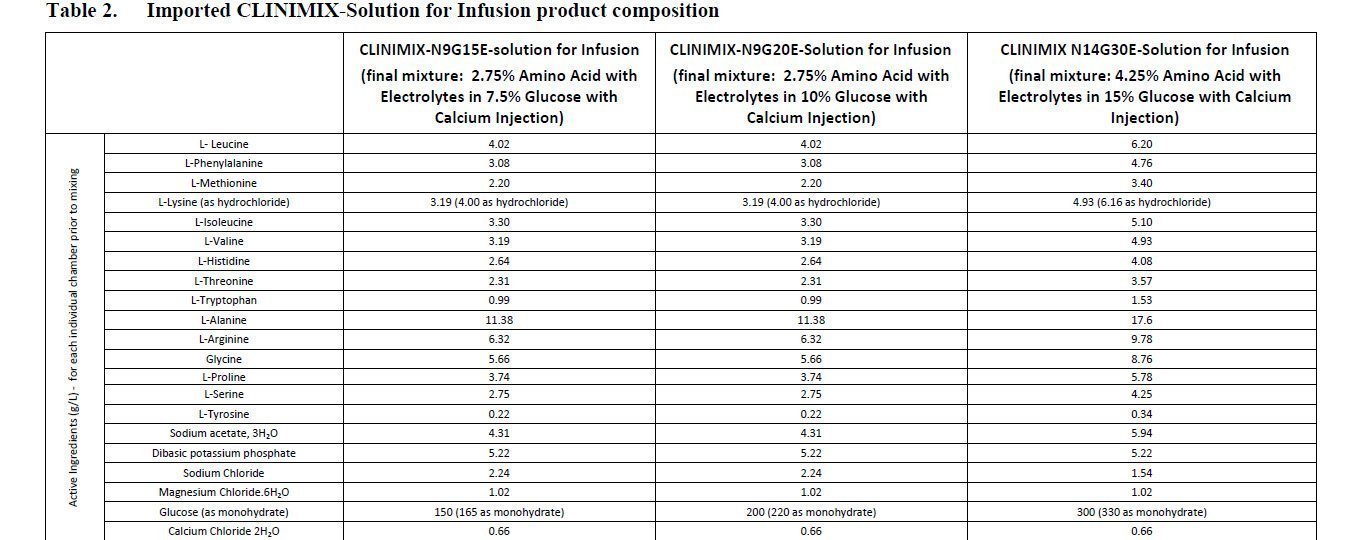
Clinimix FDA prescribing information, side effects and uses

Clinimix E Package Insert
Web The Clinimix E Dosage Can Be Adjusted Based On The Severity Of Kidney Disease, Supplementing Protein As Indicated.
Compatibility Of The Additives With The Product Must Be.
8% Amino Acids (Blend D).
Compatibility Of The Additives With The.
Related Post: