Chemical Bond Drawing
Chemical Bond Drawing - Each carbon atom has four bonds. Web drawing every bond and every atom is tedious, however, so chemists have devised several shorthand ways for writing structures. A lewis diagram shows how the valence electrons are distributed around the atoms in a molecule. Predicting bond type (metals vs. Lewis diagram of formaldehyde (ch₂o) worked example: How does molecule shape change with different numbers of bonds and electron pairs? Web to draw a bond from a single atom, simply click the atom. Use the chemical sketch tool to draw or edit a molecule. Each oxygen atom has two bonds. Web how to draw chemical bonding? Single bonds have a bond order of one, and multiple bonds with bond orders of two (a double bond) and three (a triple bond) are quite common. Once you’ve drawn a molecule, you can click the 2d to 3d button to convert the molecule into a 3d model which is then. Bond order is the number of electron pairs that. Web drawing every bond and every atom is tedious, however, so chemists have devised several shorthand ways for writing structures. Then, compare the model to real molecules! Web drawing every bond and every atom is tedious, however, so chemists have devised several shorthand ways for writing structures. Drawchemistry — is an interface created with the help of professional chemists. Ionic. Lewis diagram of the cyanide ion (cn⁻) exceptions to the octet rule. Marvinsketch allows you to draw a bond between any atoms. The structural formula editor is surround by three toolbars which contain the tools you can use in the editor. Web to draw a bond from a single atom, simply click the atom. During this course, you will view. Web when drawing the structure of a neutral organic compound, you will find it helpful to remember that. Each oxygen atom has two bonds. Lewis diagram of the cyanide ion (cn⁻) exceptions to the octet rule. Predicting bond type (metals vs. How does molecule shape change with different numbers of bonds and electron pairs? Upload a structure file or draw using a molecule editor. Web chemical bonding, any of the interactions that account for the association of atoms into molecules, ions, crystals, and other stable species that make up the familiar substances of the everyday world. The structural formula editor is surround by three toolbars which contain the tools you can use in the. It depends whether the bonds are ionic or covalent. Web represent chemical structures with the widest range of chemical bonds avialable in any software tool. Each carbon atom has four bonds. Web drawing every bond and every atom is tedious, however, so chemists have devised several shorthand ways for writing structures. This unit is part of the chemistry library. Each carbon atom has four bonds. Molview consists of two main parts, a structural formula editor and a 3d model viewer. Each hydrogen atom has one bond. During this course, you will view molecules written in all three forms. Once you’ve drawn a molecule, you can click the 2d to 3d button to convert the molecule into a 3d model. Each hydrogen atom has one bond. Each nitrogen atom has three bonds. How does molecule shape change with different numbers of bonds and electron pairs? Web 1,068,577 computed structure models (csm) chemical sketch tool. It is possible to add a bond to an empty canvas. Shared pairs of electrons are drawn as lines between atoms, while lone pairs of electrons are drawn as dots next to atoms. How does molecule shape change with different numbers of bonds and electron pairs? Web to draw a bond from a single atom, simply click the atom. This unit is part of the chemistry library. Web drawing every bond. In this case, a carbon atom is added to each end of the bond. Then, compare the model to real molecules! Valence errors are highlighted (if the associated option is enabled). It is possible to add a bond to an empty canvas. It depends whether the bonds are ionic or covalent. During this course, you will view molecules written in all three forms. Valence errors are highlighted (if the associated option is enabled). Search by structure or substructure. A lewis diagram shows how the valence electrons are distributed around the atoms in a molecule. Use the chemical sketch tool to draw or edit a molecule. Bond energies and bond lengths. Each nitrogen atom has three bonds. Web how to draw chemical bonding? 515k views 10 years ago mcat dot structures. Each carbon atom has four bonds. Each oxygen atom has two bonds. Single bonds have a bond order of one, and multiple bonds with bond orders of two (a double bond) and three (a triple bond) are quite common. Web drawing every bond and every atom is tedious, however, so chemists have devised several shorthand ways for writing structures. Bond order is the number of electron pairs that hold two atoms together. In this case, a carbon atom is added to each end of the bond. Explain how covalent bonds are formed as a result of the ability of atoms to share electrons.
How To Draw Covalent Bonds

chemical bonding Ionic and covalent compounds Britannica

Types of chemical bonding diagram. Covalent polar and nonpolar, ionic
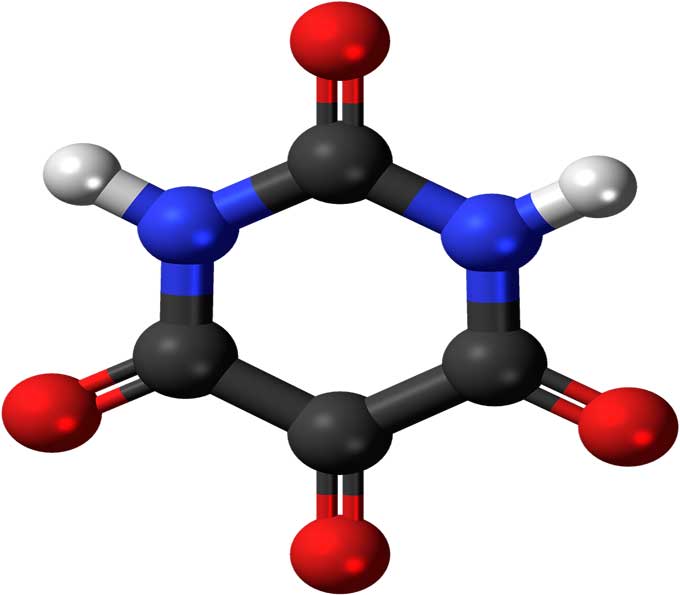
Chemical Bonding (Types, Formation, and Facts) Science4Fun
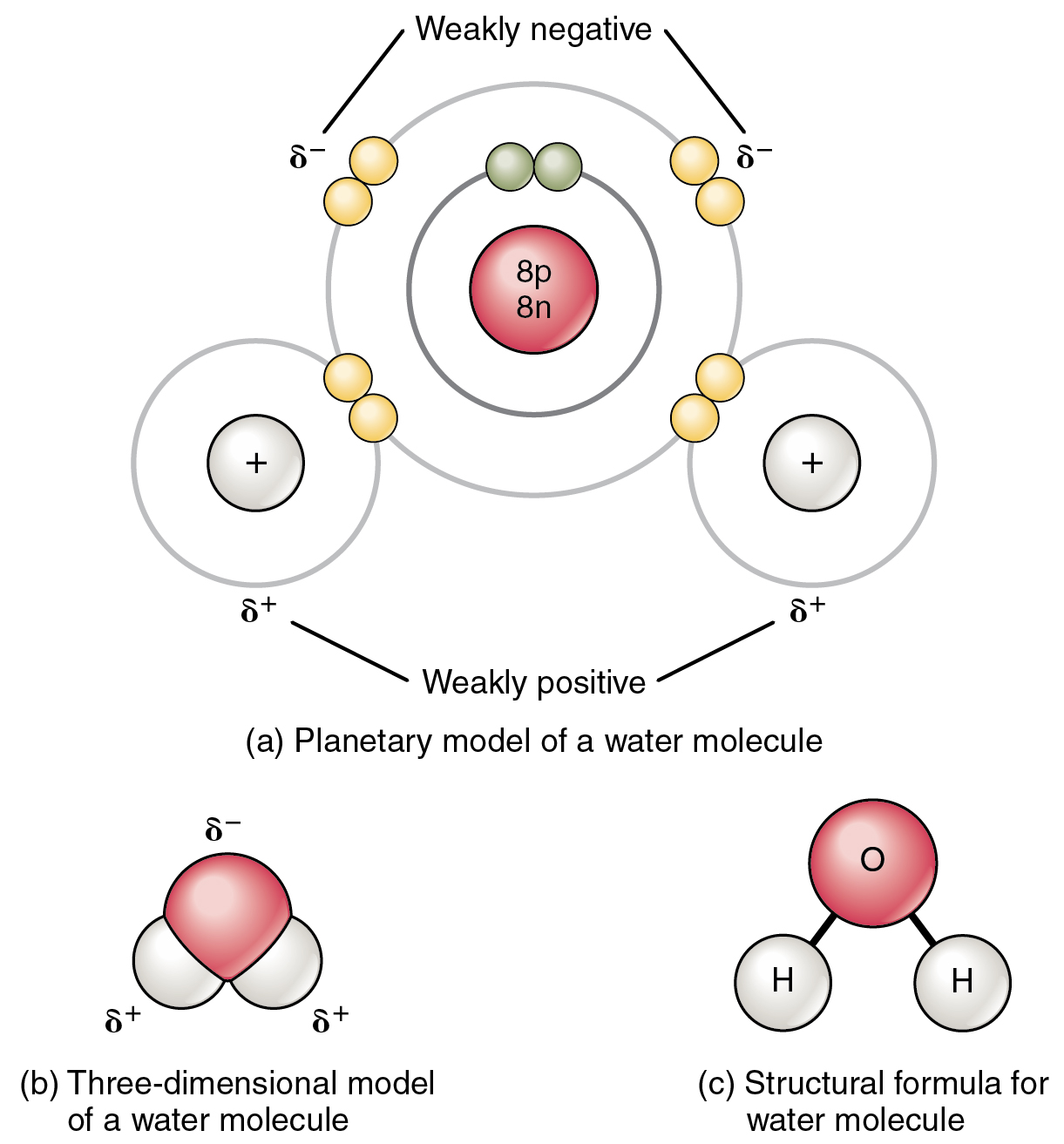
Chemical Bonds · Anatomy and Physiology
Download free photo of
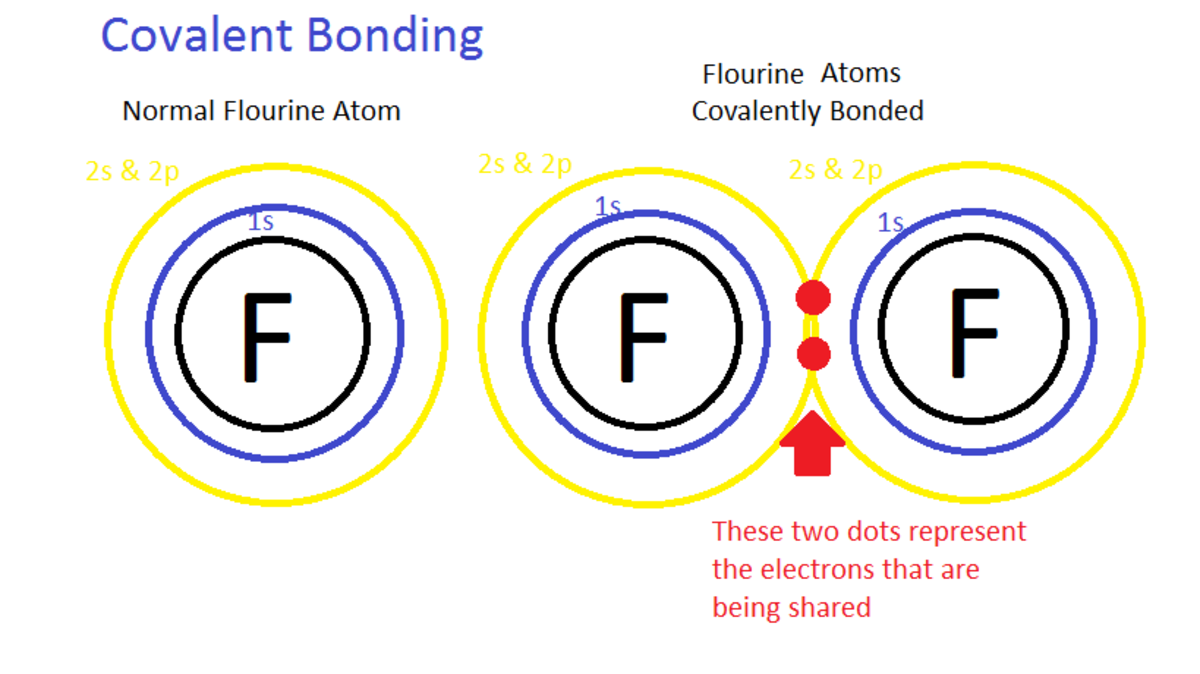
Chemical Bonding How Do Atoms Combine? What Forces Bind Atoms Together
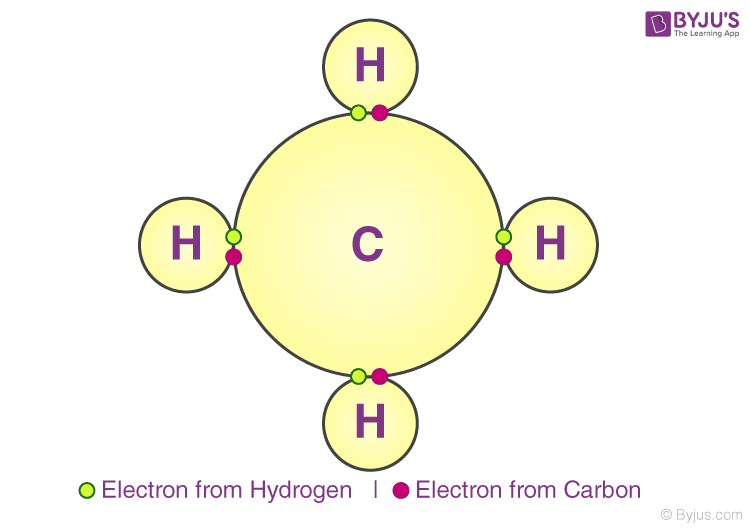
Chemical Bonding Types of Chemical Bonds, Bond Characteristics, Enthalpy

Chemical Bonding Facts for Kids

Chemical Bonds, Ionic, Covalent and Metallic AQA C2 revisechemistry.uk
Web Chemical Bonding, Any Of The Interactions That Account For The Association Of Atoms Into Molecules, Ions, Crystals, And Other Stable Species That Make Up The Familiar Substances Of The Everyday World.
A Carbon Atom Is Added At The Other End Of The Bond.
Drawchemistry Lets You Create Structural Formula Online And Save It As Either.mol Or.png.
Web Explore Molecule Shapes By Building Molecules In 3D!
Related Post: