Base Strength Chart
Base Strength Chart - Chart or notebook size available. Web 34 rows use this acids and bases chart to find the relative strength of the most common acids and bases. Examples of strong acids are hydrochloric acid. Web the relative strength of an acid or base is the extent to which it ionizes when dissolved in water. Web a strong base yields 100% (or very nearly so) of oh − and hb + when it reacts with water; Figure 2 lists a series of acids and bases. The strong bases are listed at the bottom right of the table and get weaker. Stronger acids form weaker conjugate. A weak base yields a small. Web determine at a glance the relative strengths of a host of acids and bases. If the ionization reaction is essentially complete, the acid or. Web determine at a glance the relative strengths of a host of acids and bases. The strong bases are listed at the bottom right of the table and get weaker. Web use this acids and bases chart to find the relative strength of the most common acids and bases. Web. Web determine at a glance the relative strengths of a host of acids and bases. A weak base yields a small. Web stronger acids, such as hcl, react almost completely with water, whereas weaker acids, such as acetic acid (ch 3 co 2 h), react only slightly. Figure 2 lists a series of acids and bases. Web a strong base. If the ionization reaction is essentially complete, the acid or. If the ionization reaction is essentially complete, the acid or base is termed. Web the relative strength of an acid or base is the extent to which it ionizes when dissolved in water. The strong bases are listed at the bottom right of the table and get weaker. Chart or. Web determine at a glance the relative strengths of a host of acids and bases. Chart or notebook size available. If the ionization reaction is essentially complete, the acid or base is termed. Organic chemists customarily compare the strength of bases using the. Stronger acids form weaker conjugate. Web use this acids and bases chart to find the relative strength of the most common acids and bases. Web the greater the ability of a species to accept a h+ from another species, the greater its base strength. A weak base yields a small. Chart or notebook size available. The strong bases are listed at the bottom right of. Examples of strong acids are hydrochloric acid. Web stronger acids, such as hcl, react almost completely with water, whereas weaker acids, such as acetic acid (ch 3 co 2 h), react only slightly. Figure 2 lists a series of acids and bases. Organic chemists customarily compare the strength of bases using the. Web use this acids and bases chart to. Web stronger acids, such as hcl, react almost completely with water, whereas weaker acids, such as acetic acid (ch 3 co 2 h), react only slightly. Web assess the relative strengths of acids and bases according to their ionization constants. Web use this acids and bases chart to find the relative strength of the most common acids and bases. Chart. Web use this acids and bases chart to find the relative strength of the most common acids and bases. Figure \(\pageindex{1}\) lists several strong bases. Web assess the relative strengths of acids and bases according to their ionization constants; Web the greater the ability of a species to accept a h+ from another species, the greater its base strength. The. Web the greater the ability of a species to accept a h+ from another species, the greater its base strength. Web assess the relative strengths of acids and bases according to their ionization constants. Web a strong base yields 100% (or very nearly so) of oh − and hb + when it reacts with water; Web stronger acids, such as. Chart or notebook size available. Figure \(\pageindex{1}\) lists several strong bases. Examples of strong acids are hydrochloric acid. Figure 2 lists a series of acids and bases. Web use this acids and bases chart to find the relative strength of the most common acids and bases. Figure 2 lists a series of acids and bases. Web the relative strength of an acid or base is the extent to which it ionizes when dissolved in water. Chart or notebook size available. Web the relative strength of an acid or base is the extent to which it ionizes when dissolved in water. Web the greater the ability of a species to accept a h+ from another species, the greater its base strength. Examples of strong acids are hydrochloric acid. A weak base yields a small. Web assess the relative strengths of acids and bases according to their ionization constants; If the ionization reaction is essentially complete, the acid or base is termed. Organic chemists customarily compare the strength of bases using the. Web 34 rows use this acids and bases chart to find the relative strength of the most common acids and bases. Stronger acids form weaker conjugate. Web use this acids and bases chart to find the relative strength of the most common acids and bases. Web use this acids and bases chart to find the relative strength of the most common acids and bases. Web assess the relative strengths of acids and bases according to their ionization constants. Web stronger acids, such as hcl, react almost completely with water, whereas weaker acids, such as acetic acid (ch 3 co 2 h), react only slightly.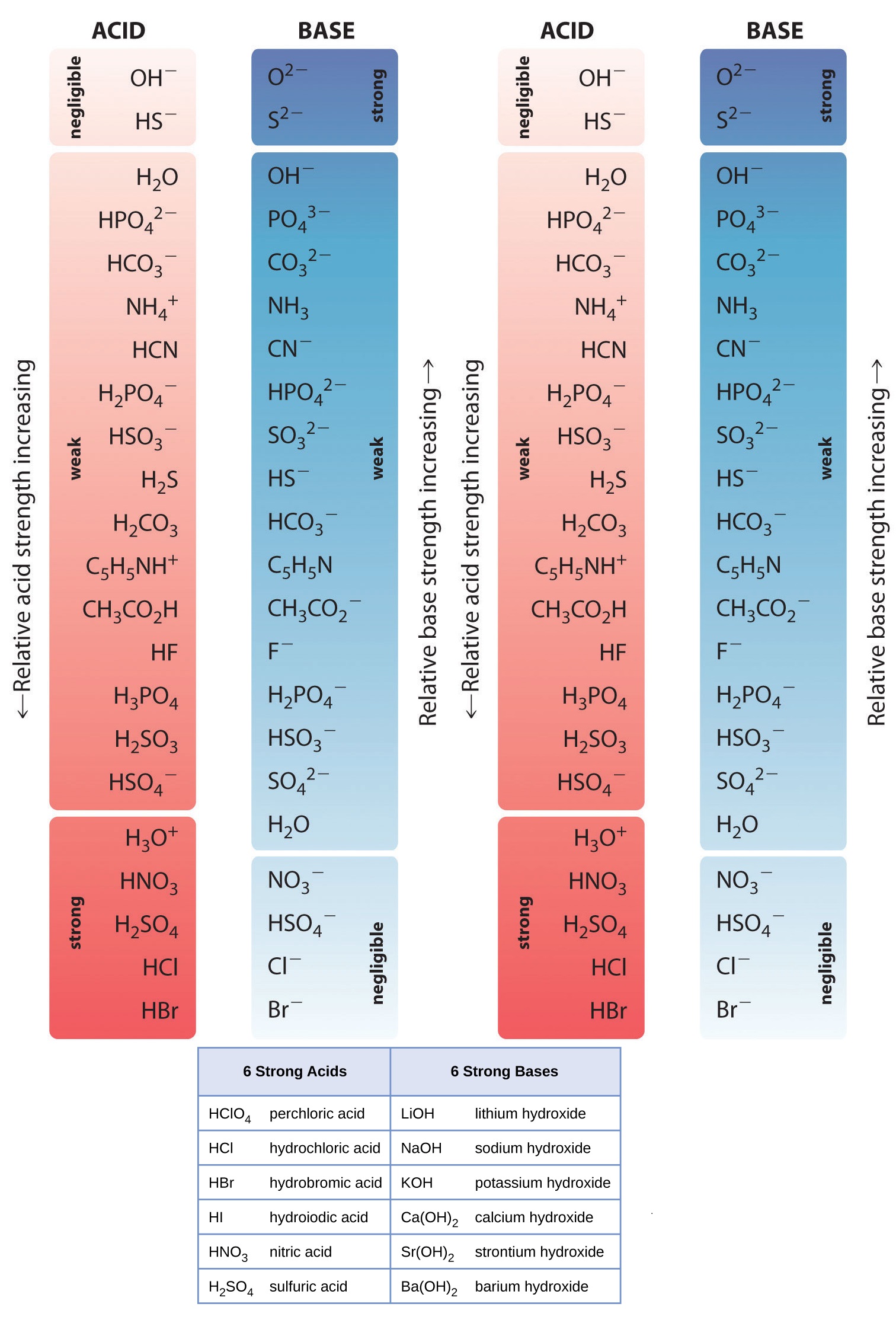
List of Strong Acids & Bases in Order StudyPK
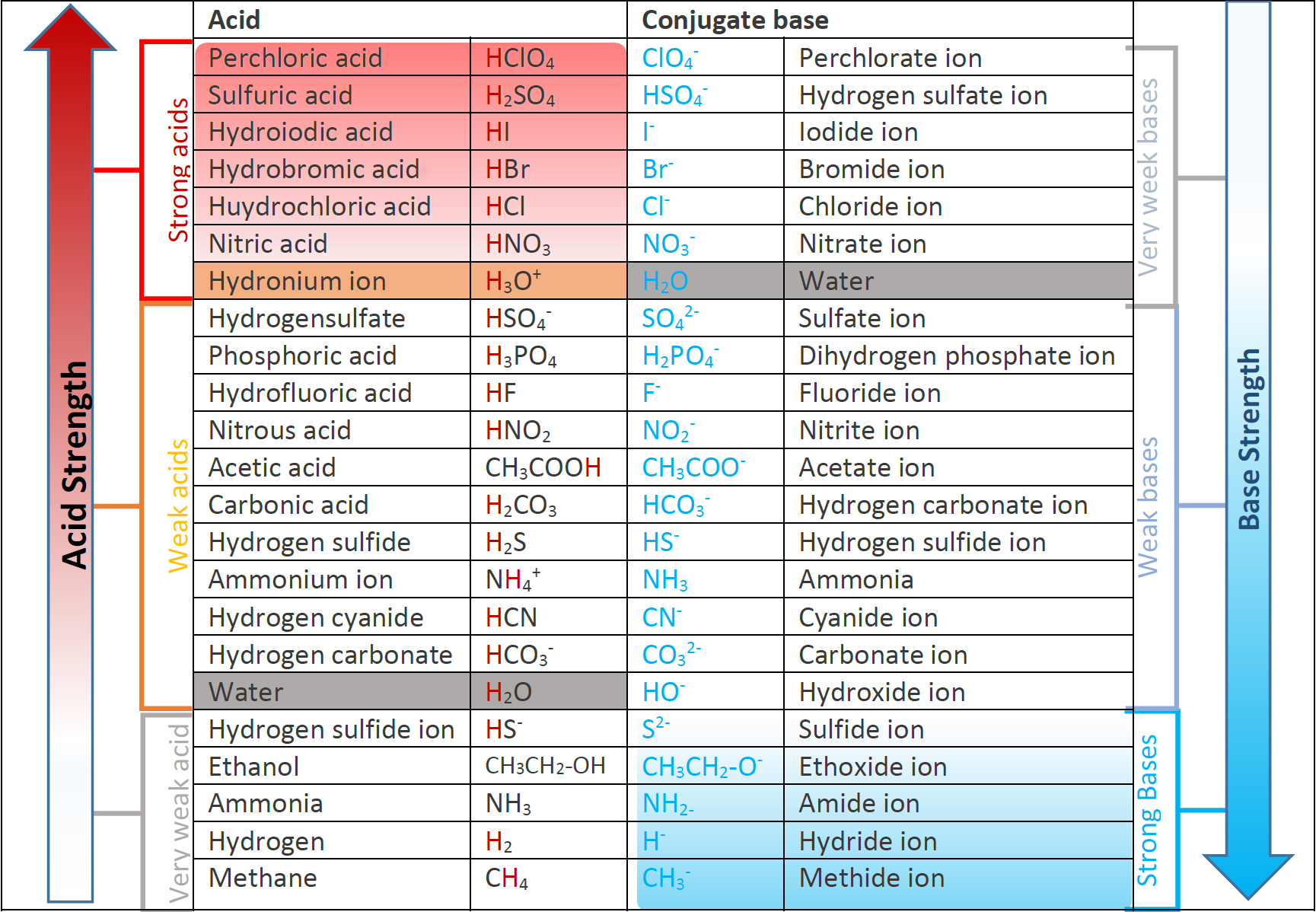
6.3 Strength of acids and bases Chemistry LibreTexts
Acids And Bases List Strength
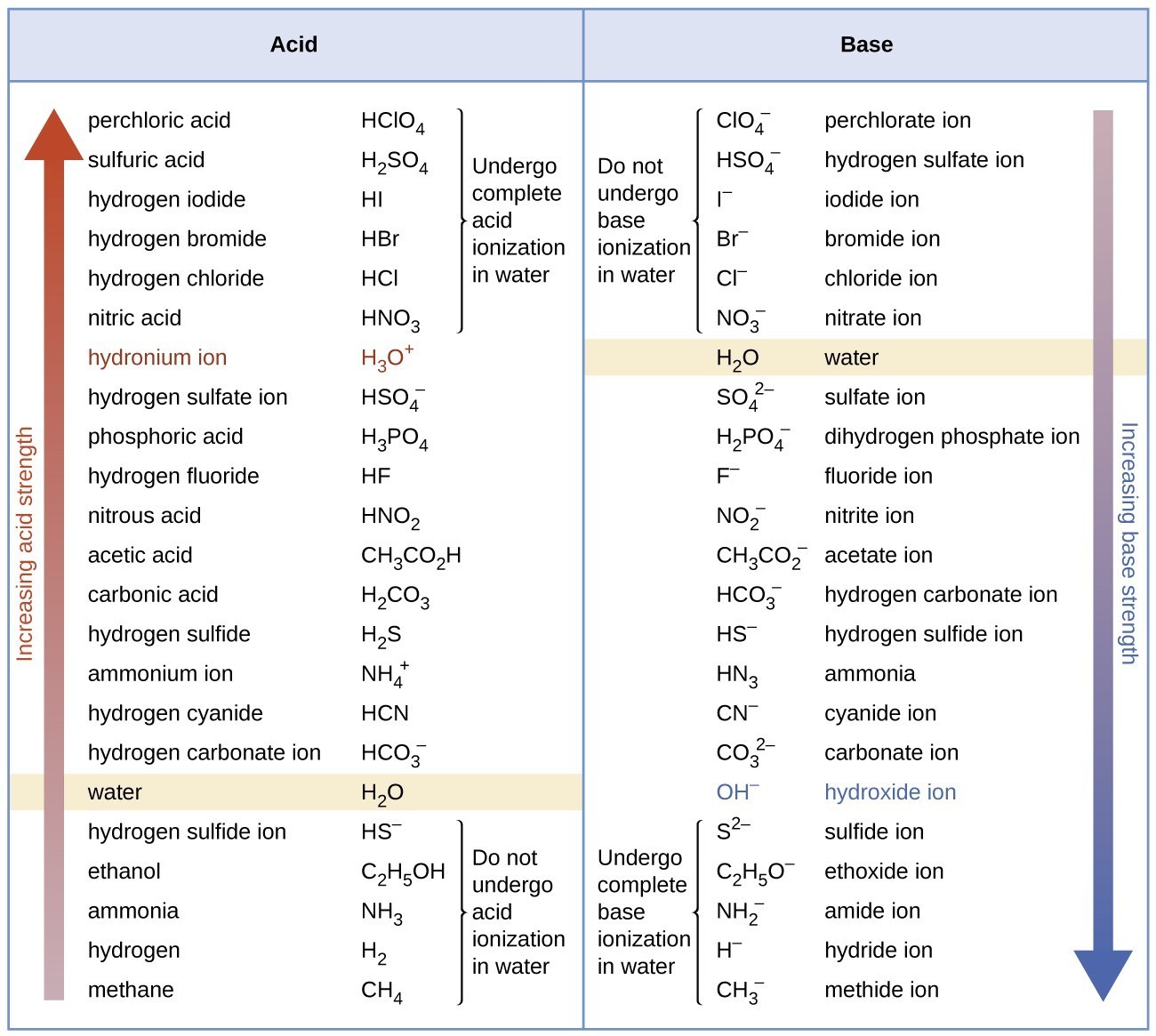
Relative Strengths of Acids and Bases General Chemistry
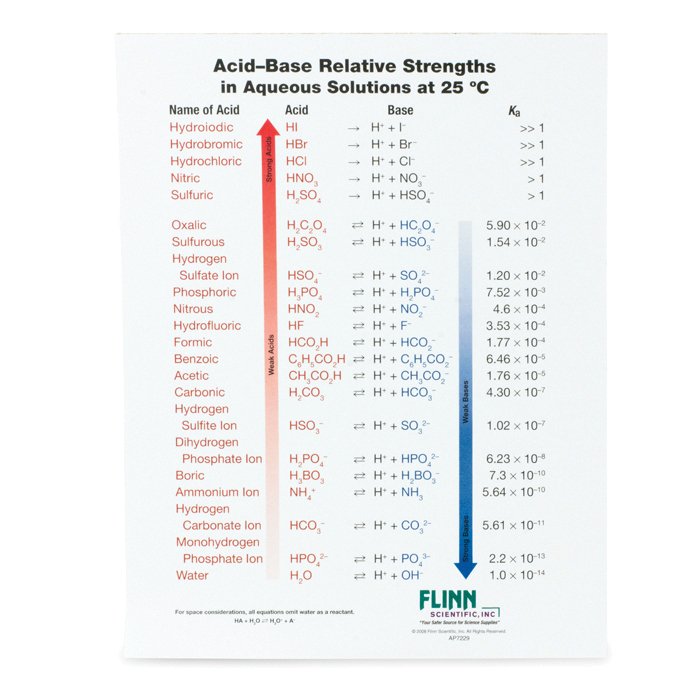
AcidBase Strength Charts for Chemistry
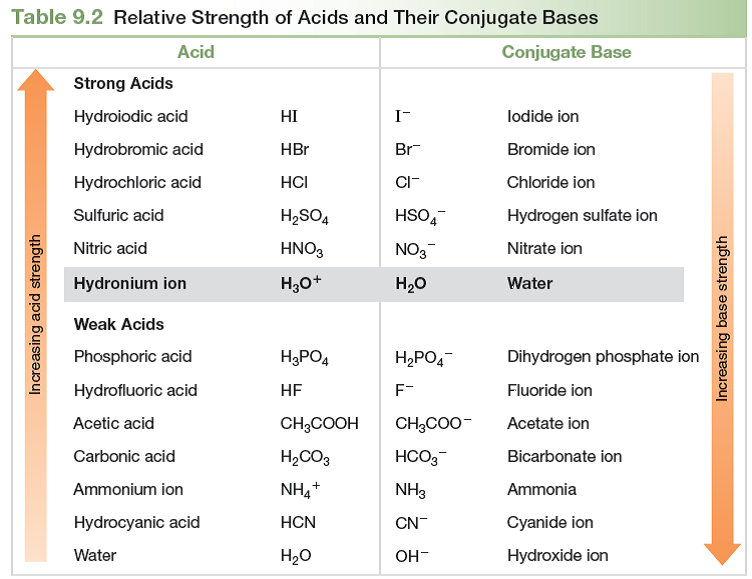
Acid And Base Strength Chart
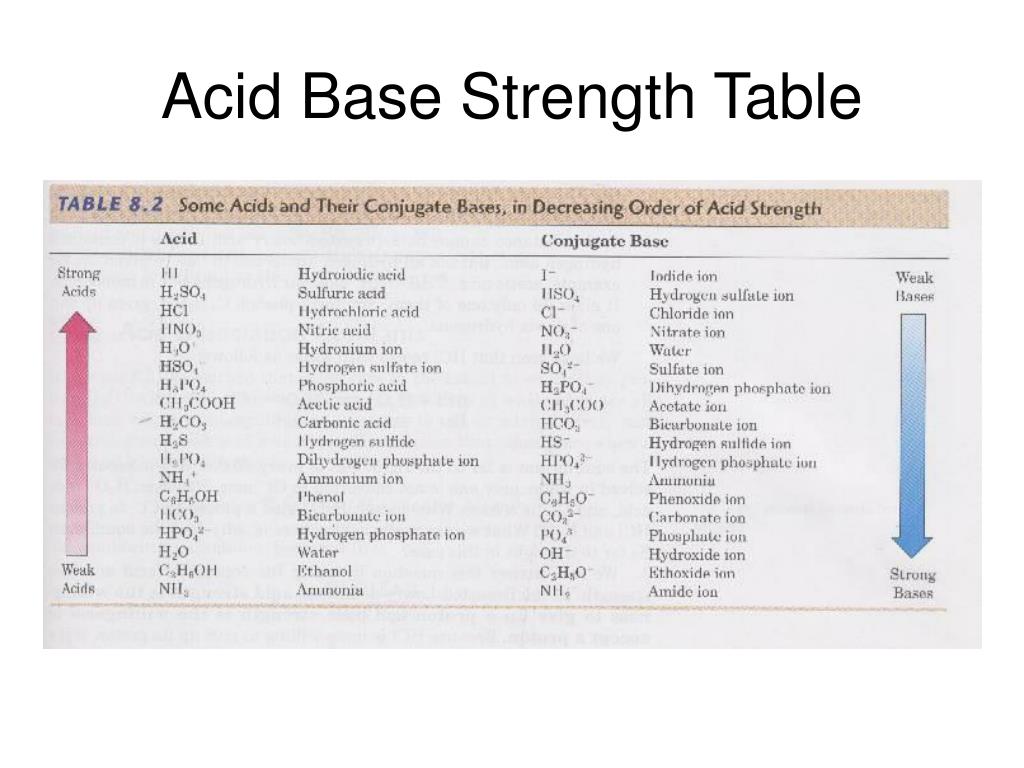
Acid Base Strength Chart
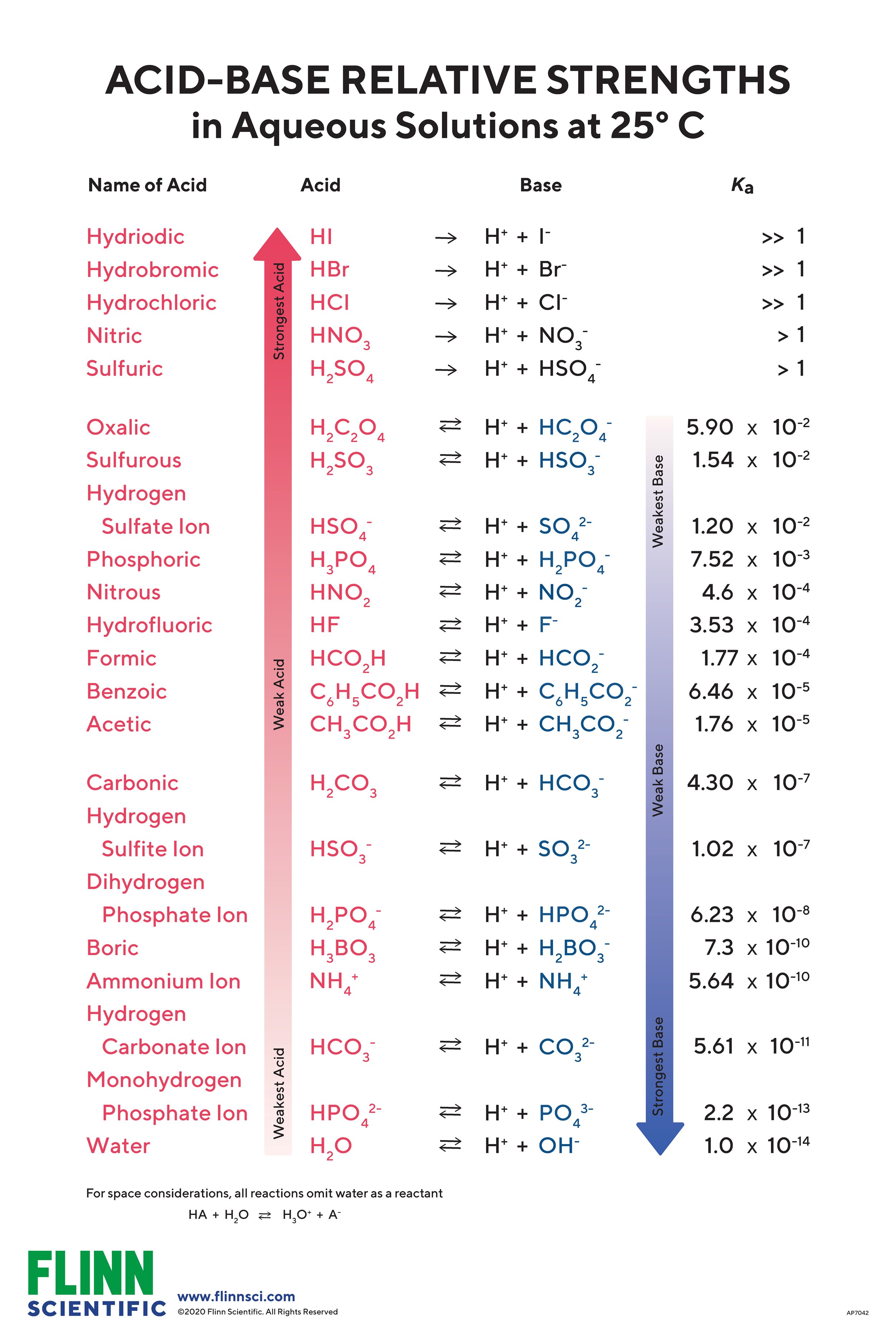
AcidBase Strength Chart

Acid Base Strength Chart
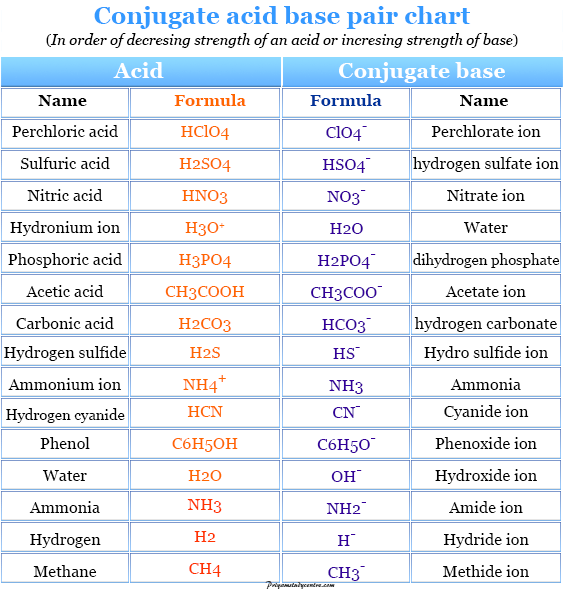
Acids and Bases Definition, Concept, Theory, Examples
Web A Strong Base Yields 100% (Or Very Nearly So) Of Oh − And Hb + When It Reacts With Water;
Figure \(\Pageindex{1}\) Lists Several Strong Bases.
The Exact Strength Of A Given.
If The Ionization Reaction Is Essentially Complete, The Acid Or.
Related Post:
