Atomic Radius Chart Periodic Table
Atomic Radius Chart Periodic Table - First and second ionization energy. Web atomic radius (ionic radius) atomic radius is the distance from the nucleus to the outermost stable electron while ionic radius is half the distance between two atomic nuclei that are just touching each other. Because of these two trends, the largest atoms are found in the lower left corner of the periodic table, and the smallest are found in the upper right corner (figure \(\pageindex{4}\)). As there are no physical existence of orbital in atoms, it is difficult to measure the atomic radius. This is due to the way electrons form shells around the nucleus. And the increasing nuclear charge pulls the electron clouds inwards, making the atomic radii smaller. The atomic radius of atoms generally increases from top to. Pdf without crop marks | pdf with crop marks. Web atomic radius is determined as half the distance between the nuclei of two identical atoms bonded together. These related values display the same trend in. Because of these two trends, the largest atoms are found in the lower left corner of the periodic table, and the smallest are found in the upper right corner (figure \(\pageindex{4}\)). The atomic radius of a chemical element is a measure of the size of its atom, usually the mean or typical distance from the center of the nucleus to. Periodic trends, arising from the arrangement of the periodic table, provide chemists with an invaluable tool to. Ionic radii are also available. Web atomic radius trends on periodic table. The atomic radius of a chemical element is a measure of the size of its atom, usually the mean or typical distance from the center of the nucleus to the outermost. Diagram of a helium atom, showing the electron probability density as shades of gray. As there are no physical existence of orbital in atoms, it is difficult to measure the atomic radius. Visualize trends, 3d orbitals, isotopes, and mix compounds. Web the periodic table of the elements (including atomic radius) element name. Want to join the conversation? Web this table shows how the atom size, and atomic radius values change as you move horizontally and vertically across the periodic table. The atomic radius of a chemical element is a measure of the size of its atom, usually the mean or typical distance from the center of the nucleus to the outermost isolated electron. Pdf without crop marks. Web major periodic trends include: Web explore how atomic radius changes with atomic number in the periodic table of elements via interactive plots. Web atomic radius trends on periodic table. The atomic radius of atoms generally decreases from left to right across a period. Web the latest release of the periodic table (dated 4 may 2022) includes the most recent. Periodic trends, arising from the arrangement of the periodic table, provide chemists with an invaluable tool to. Web how atomic radius is defined, and trends across a period and down a group. The atomic radius of atoms generally decreases from left to right across a period. Atoms decrease in size across the period and increase in size down the group.. Web atomic radius trends on periodic table. Although more electrons are being added to atoms, they are at similar distances to the nucleus; Going across a period, the main group elements tend to decrease in atomic radius due to the increased nuclear charge. Web the periodic table of the elements (including atomic radius) element name. Web interactive periodic table showing. Ionic radii are also available. Web interactive periodic table showing names, electrons, and oxidation states. Web how atomic radius is defined, and trends across a period and down a group. First and second ionization energy. Web in the periodic table, atomic radii decrease from left to right across a row and increase from top to bottom down a column. Atoms decrease in size across the period and increase in size down the group. Web major periodic trends include: First and second ionization energy. Web the periodic table is a graphic description of the periodic law, which states that the properties and atomic structures of the chemical elements are a periodic function of their atomic number. Web atomic radius trends. Web explore how atomic radius changes with atomic number in the periodic table of elements via interactive plots. Web the periodic table is a graphic description of the periodic law, which states that the properties and atomic structures of the chemical elements are a periodic function of their atomic number. Web the atomic radius is the size of the atom,. The atomic radius of atoms generally increases from top to. Web atomic radius is determined as half the distance between the nuclei of two identical atoms bonded together. Web the atomic radius of a chemical element is the distance from the center of the nucleus to the outermost shell of an electron. As there are no physical existence of orbital in atoms, it is difficult to measure the atomic radius. Atomic radii (empirical) atomic radii (absolute) atomic radii (density cutoff) covalent radii revisited (2008 values) covalent radii (molecular single bond) Web how atomic radius is defined, and trends across a period and down a group. Web select from the following links to see visual periodicity representations for atomic radii, covalent radii, and van der waals radii. Web in the periodic table, atomic radii decrease from left to right across a row and increase from top to bottom down a column. Web the relative size of the atoms follows a set of trends on the periodic table. Because of these two trends, the largest atoms are found in the lower left corner of the periodic table, and the smallest are found in the upper right corner (figure \(\pageindex{4}\)). Web this table shows how the atom size, and atomic radius values change as you move horizontally and vertically across the periodic table. Periodic trends, arising from the arrangement of the periodic table, provide chemists with an invaluable tool to. Web atomic radius of all the elements are mentioned in the chart below. And the increasing nuclear charge pulls the electron clouds inwards, making the atomic radii smaller. Web major periodic trends include: Below mentioned radii are the van der waals radius in picometer (pm)).
Atomic Radius of Elements The Periodic Table
/accurate-illustration-of-the-periodic-table-82020791-57cc76f23df78c71b66efbd7.jpg)
Atomic Radius Table Pdf Elcho Table
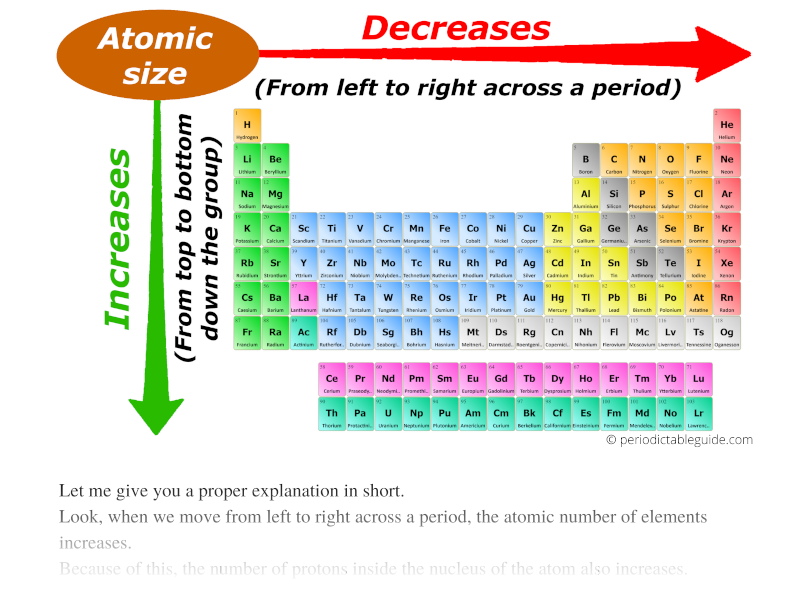
Atomic radius chart mindsstorm
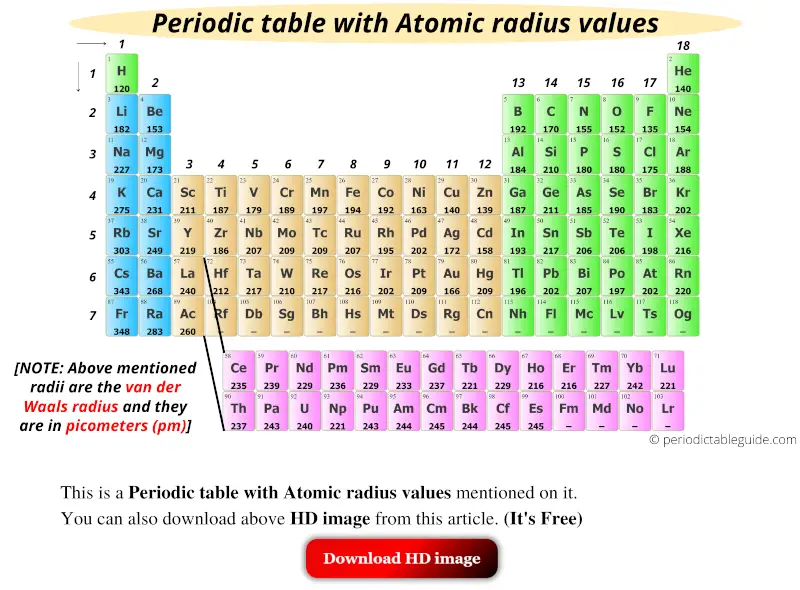
Get the Periodic table with Atomic radius values (Img+Chart)
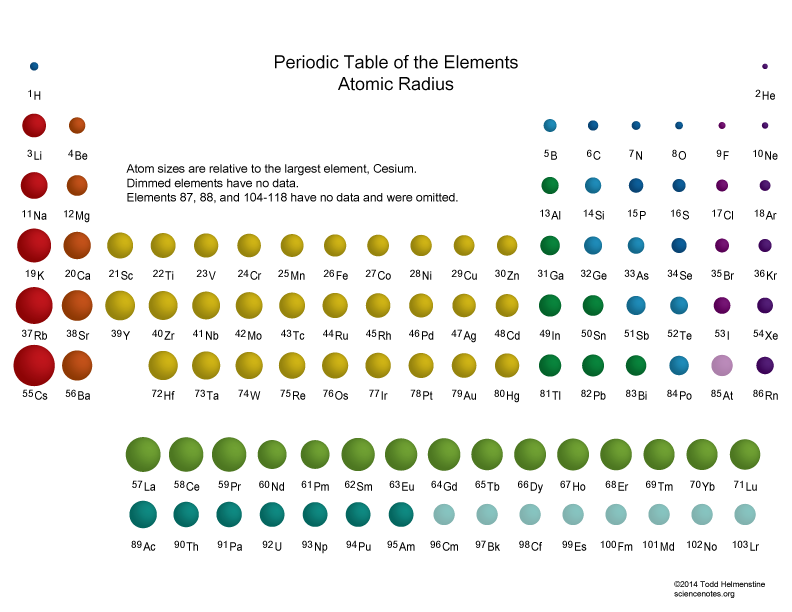
Atomic Radius Trends of the Periodic Table
.png)
Periodic Table Of The Elements Atomic Radius vrogue.co
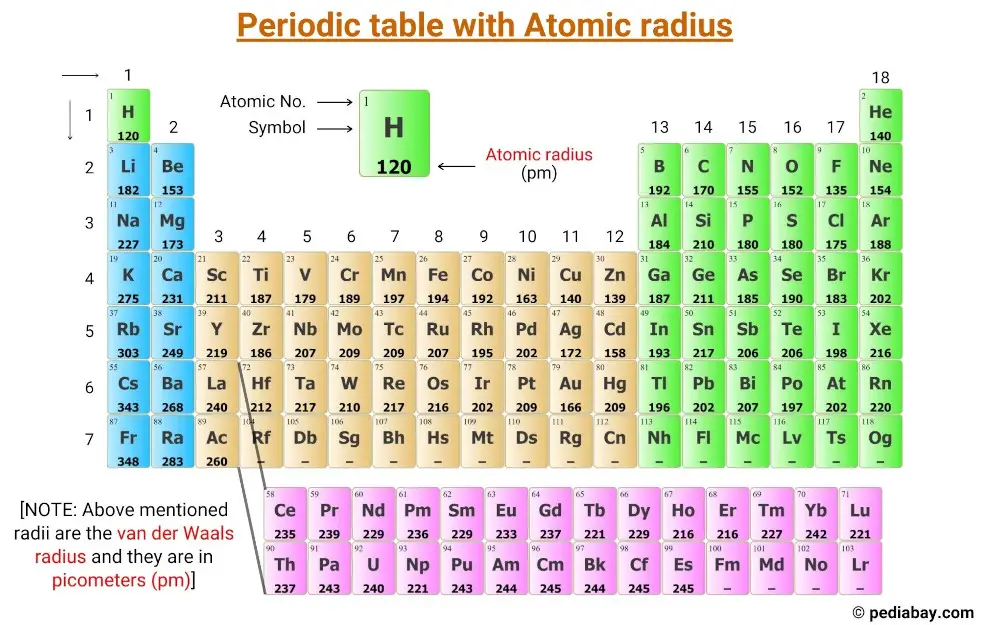
Atomic Radius of Elements (With Periodic table Chart) Pediabay
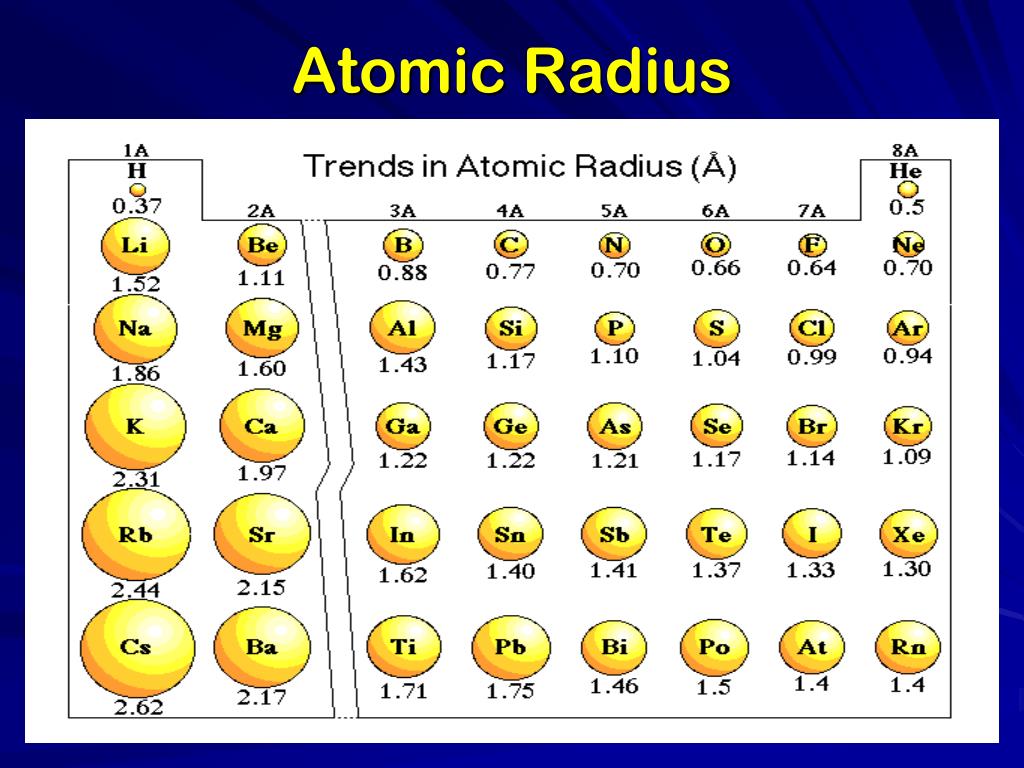
PPT Periodic Table PowerPoint Presentation, free download ID2617702

Atomic Radius of Elements
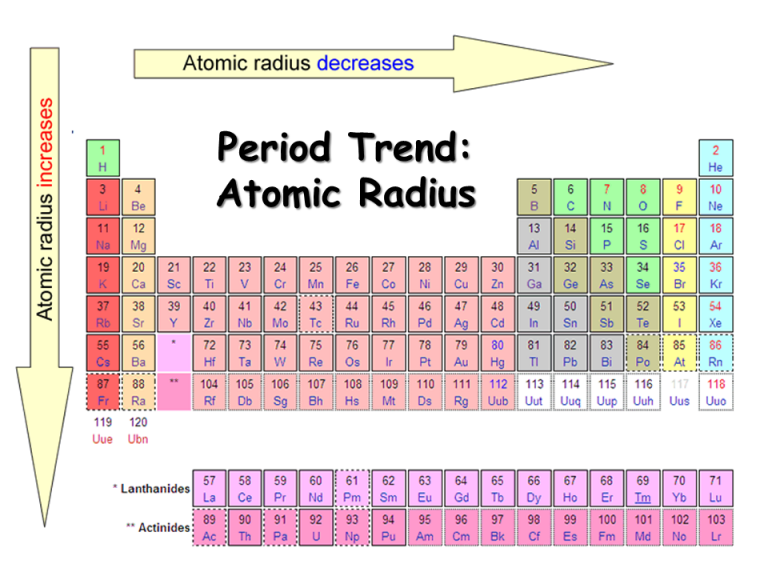
Atomic Radius NEETLab
Atoms Decrease In Size Across The Period And Increase In Size Down The Group.
Web Explore How Atomic Radius Changes With Atomic Number In The Periodic Table Of Elements Via Interactive Plots.
Web Atomic Radius Trends On Periodic Table.
Web The Latest Release Of The Periodic Table (Dated 4 May 2022) Includes The Most Recent Abridged Standard Atomic Weight Values Released By The Iupac Commission On Isotopic Abundances And Atomic Weights ( Ciaaw ), Compiled As Part Of The 2021 Table Of Standard Atomic Weights 2021.
Related Post: