Atom Drawing Of Hydrogen
Atom Drawing Of Hydrogen - Web elements are shown from atomic number 1 (hydrogen) up to 94 (plutonium). Web two hydrogen atoms can combine by donating each of their electrons into a single covalent bond, depicted on the right as the area where the gray clouds around each hydrogen atom overlap. Web the hydrogen atom consists of a single negatively charged electron that moves about a positively charged proton. However, it's easy to determine the configuration of electrons for heavier elements by making a chart. Isotopes of hydrogen complete table of nuclides 5.5k views 8 years ago. Chemistry (zumdahl and decoste) 12: 114k views 11 years ago. Identify the physical significance of each of the quantum numbers ( n, l, m) of the hydrogen atom. A very simple drawing of an hydrogen atom. This permits the shapes of the electron waves to be more complicated. Then play a game to test your ideas! E ( n) = − 1 n 2 ⋅ 13.6 ev. The most common element in the universe is hydrogen, a gas that makes up about 99% of the universe’s known mass 1. The energy transition results in a balmer. The diameter of a hydrogen atom is roughly 100,000 times larger than a proton. Chemistry (zumdahl and decoste) 12: Try out different models by shooting light at the atom. Then play a game to test your ideas! The energy transition results in a balmer series line in an emission spectrum. Both these effects are of the same order of magnitude, and must be treated together in any realistic treatment of the atomic structure. The most common element in the universe is hydrogen, a gas that makes up about 99% of the universe’s known mass 1. Niels bohr, danish physicist, used the planetary model of the atom to explain the atomic. The energy transition results in a balmer series line in an emission spectrum. Visible spectrum of atomic hydrogen. We’ll use a bohr diagram to. Check how the prediction of the model matches the experimental results. E ( n) = − 1 n 2 ⋅ 13.6 ev. Try out different models by shooting light at the atom. In the covalent bond, the electron pair is shared between the two hydrogen atoms. Web how did scientists figure out the structure of atoms without looking at them? Web hydrogen atom, 1 h; Check how the prediction of the model matches the experimental results. We’ll use a bohr diagram to. The quantum mechanical model of the atom. A hydrogen discharge tube is a slim tube containing hydrogen gas at low pressure with an electrode at each end. 114k views 11 years ago. Web how did scientists figure out the structure of atoms without looking at them? E ( n) = − 1 n 2 ⋅ 13.6 ev. In bohr’s model, the electron is pulled around the proton in a perfectly circular orbit by an attractive coulomb force. Web build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. Lithium is the first element in which an additional. Visible. Web how did scientists figure out the structure of atoms without looking at them? Quantum numbers for the first four shells. 14k views 1 year ago. The diameter of a hydrogen atom is roughly 100,000 times larger than a proton. In the covalent bond, the electron pair is shared between the two hydrogen atoms. Web the hydrogen atom consists of a single negatively charged electron that moves about a positively charged proton. Check how the prediction of the model matches the experimental results. Web elements are shown from atomic number 1 (hydrogen) up to 94 (plutonium). The atomic spectrum of hydrogen. Niels bohr, danish physicist, used the planetary model of the atom to explain. First, in an atom the electron occupies all three dimensions of ordinary space. Web how did scientists figure out the structure of atoms without looking at them? The diameter of a hydrogen atom is roughly 100,000 times larger than a proton. For the h2 structure use the periodic table to find the total number of. 14k views 1 year ago. 114k views 11 years ago. The atomic spectrum of hydrogen. A very simple drawing of an hydrogen atom. Try out different models by shooting light at the atom. For the h2 structure use the periodic table to find the total number of. Atomic theory and quantum mechanics. In bohr’s model, the electron is pulled around the proton in a perfectly circular orbit by an attractive coulomb force. The quantum mechanical model of the atom. Web the hydrogen atom consists of a single negatively charged electron that moves about a positively charged proton. Check how the prediction of the model matches the experimental results. Bohr's model calculated the following energies for an electron in the shell, n. Bohr's model of hydrogen is based on the nonclassical assumption that electrons travel in specific shells, or orbits, around the nucleus. Web hydrogen atom, 1 h; Electron waves in the hydrogen atom. Quantum numbers for the first four shells. We’ll use a bohr diagram to.
Hydrogen atom Bohr model stock vector. Illustration of structure

Hydrogen atom diagram concept illustration Stock Vector Image & Art Alamy

Describe Bohr’s model of the hydrogen atom. bitWise Academy

Hydrogen atom diagram concept Royalty Free Vector Image
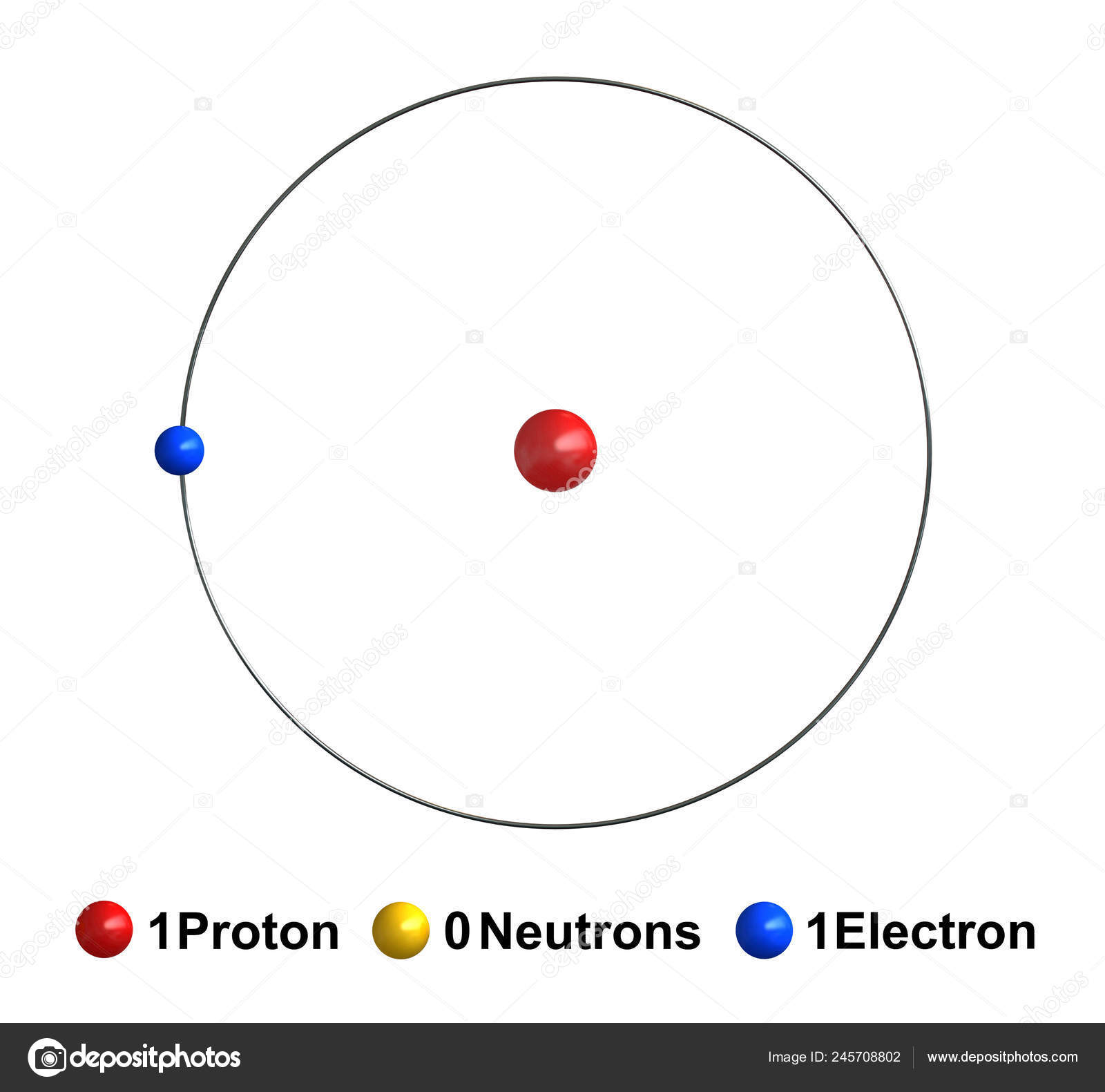
Render Atom Structure Hydrogen Isolated White Backgroun Stock Photo by

Diagram Representation Of The Element Hydrogen Stock Vector Image
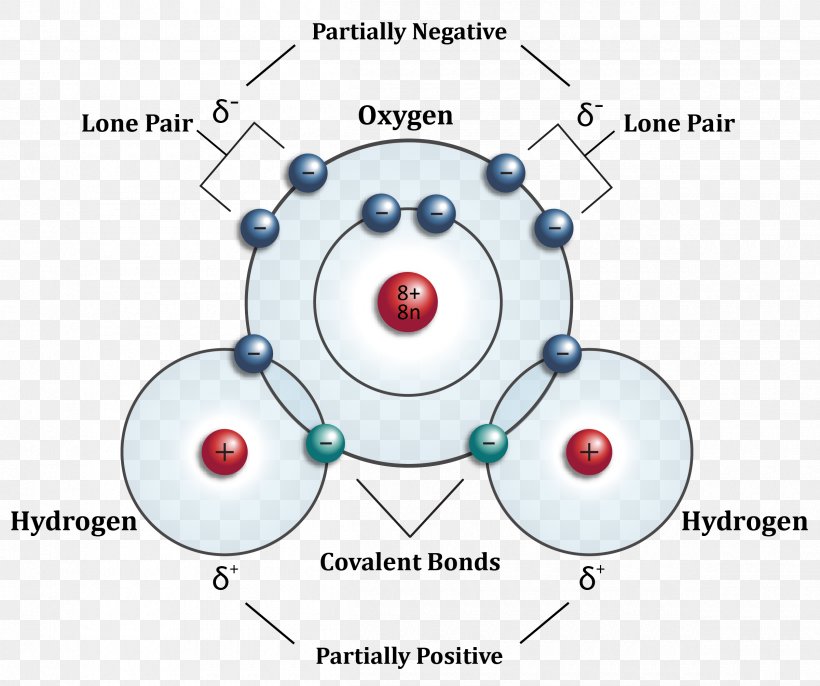
Hydrogen Atom Diagram

Diagram representation element hydrogen Royalty Free Vector

How to Learn About the Chemistry of the Hydrogen Atom 12 Steps

Hydrogen atom on white background Royalty Free Vector Image
Lithium Is The First Element In Which An Additional.
A Hydrogen Discharge Tube Is A Slim Tube Containing Hydrogen Gas At Low Pressure With An Electrode At Each End.
However, It's Easy To Determine The Configuration Of Electrons For Heavier Elements By Making A Chart.
Web Bohr’s Theory Explained The Atomic Spectrum Of Hydrogen And Established New And Broadly Applicable Principles In Quantum Mechanics.
Related Post: