Acid Strength Chart
Acid Strength Chart - Keep reading to learn all about the chemistry behind acid strength. In the ethyl anion, the negative charge is borne by carbon, while in the methylamine anion and methoxide anion the. Web a reactivity series, or acid strength chart, provides a visual representation of the relative strength of various acids. Each acid and each base has an associated ionization constant that corresponds to its acid or base strength. Web accordingly, the acid strength is defined by the concentration of undissociated acid molecules and hydronium ions. Table \(\pageindex{1}\) lists several strong acids. Assess the relative strengths of acids and bases according to their ionization constants. The more stable (weaker) the conjugate base, the stronger the acid. Chart or notebook size available. Web use this acids and bases chart to find the relative strength of the most common acids and bases. This information can be used to predict the outcome of reactions between acids and other substances, such as bases and metals. The first six acids in figure 14.3.3 are the most common strong acids. By andy brunning september 28, 2016. A guide to acids, acid strength, and concentration. Even if you’re not a chemist, you’ll doubtless remember learning about acids. Web a strong acid yields 100% (or very nearly so) of \(\ce{h3o+}\) and \(\ce{a^{−}}\) when the acid ionizes in water. Web acid with values less than one are considered weak. Web use this acids and bases chart to find the relative strength of the most common acids and bases. And how does ph fit in with these? Acid strength increases. Table \(\pageindex{1}\) lists several strong acids. Web accordingly, the acid strength is defined by the concentration of undissociated acid molecules and hydronium ions. Acid and base ionization constants. Assess the relative strengths of acids and bases according to their ionization constants. Acid strength increases down a group and increases from left to right across a period. By the end of this section, you will be able to: Web a reactivity series, or acid strength chart, provides a visual representation of the relative strength of various acids. If the ionization reaction is essentially complete, the acid or base is termed strong; Web a strong acid yields 100% (or very nearly so) of \(\ce{h3o+}\) and \(\ce{a^{−}}\) when the. Acid or base strength is a measure of how readily the molecule ionizes in water. The more stable (weaker) the conjugate base, the stronger the acid. And how does ph fit in with these? The strong bases are listed at the bottom right of the table and get weaker as we move to the top of the table. This graphic. Acid strength depends on a variety of chemical factors, including electronegativity, atomic radius, and resonance. The first six acids in figure 14.3.3 are the most common strong acids. Web acid strength is the tendency of an acid, symbolised by the chemical formula, to dissociate into a proton, +, and an anion,. Web use this acids and bases chart to find. Web use this acids and bases chart to find the relative strength of the most common acids and bases. Acid strength increases down a group and increases from left to right across a period. Table \(\pageindex{1}\) lists several strong acids. Chart or notebook size available. By the end of this section, you will be able to: By andy brunning september 28, 2016. Stronger acids form weaker conjugate bases, and weaker acids. Web acids and bases behave differently in solution based on their strength. Web a strong acid yields 100% (or very nearly so) of \(\ce{h3o+}\) and \(\ce{a^{−}}\) when the acid ionizes in water. And how does ph fit in with these? Acid strength increases down a group and increases from left to right across a period. Even if you’re not a chemist, you’ll doubtless remember learning about acids back in school. Web acid strength is the tendency of an acid, symbolised by the chemical formula, to dissociate into a proton, +, and an anion,. Web what’s the diference between acid strength. Acid strength depends on a variety of chemical factors, including electronegativity, atomic radius, and resonance. Web a strong acid yields 100% (or very nearly so) of \(\ce{h3o+}\) and \(\ce{a^{−}}\) when the acid ionizes in water. They’re routinely described as strong or weak, concentrated or dilute. A weak acid does not completely ionize in water, yielding only small amounts of \(\ce{h3o+}\). Examples of strong acids are hydrochloric acid. The strong bases are listed at the bottom right of the table and get weaker as we move to the top of the table. If relatively little ionization occurs, the acid or base is weak. A weak acid does not completely ionize in water, yielding only small amounts of \(\ce{h3o+}\) and \(\ce{a^{−}}\). Stronger acids form weaker conjugate bases, and weaker acids. Topics covered in other articles. Web a strong acid yields 100% (or very nearly so) of \(\ce{h3o+}\) and \(\ce{a^{−}}\) when the acid ionizes in water. If the ionization reaction is essentially complete, the acid or base is termed strong; Web the key to understanding this trend is to consider the hypothetical conjugate base in each case: While the weak acid can be estimated via the equilibrium constant ( keq ), it is constant for a dilute solution, and the. The acid and base in a given row are conjugate to each other. The first six acids in figure 14.3.3 are the most common strong acids. Chart or notebook size available. Visit byju's to learn more about it. Table \(\pageindex{1}\) lists several strong acids. Web use this acids and bases chart to find the relative strength of the most common acids and bases.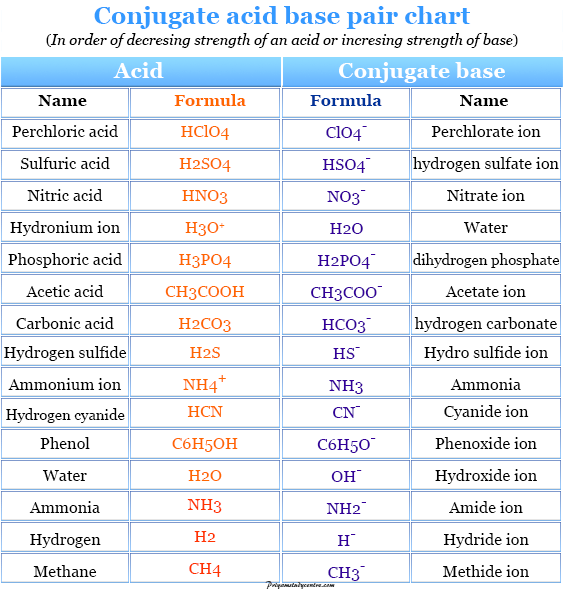
Conjugate Acid Base pair Definition, Concept, Examples, List
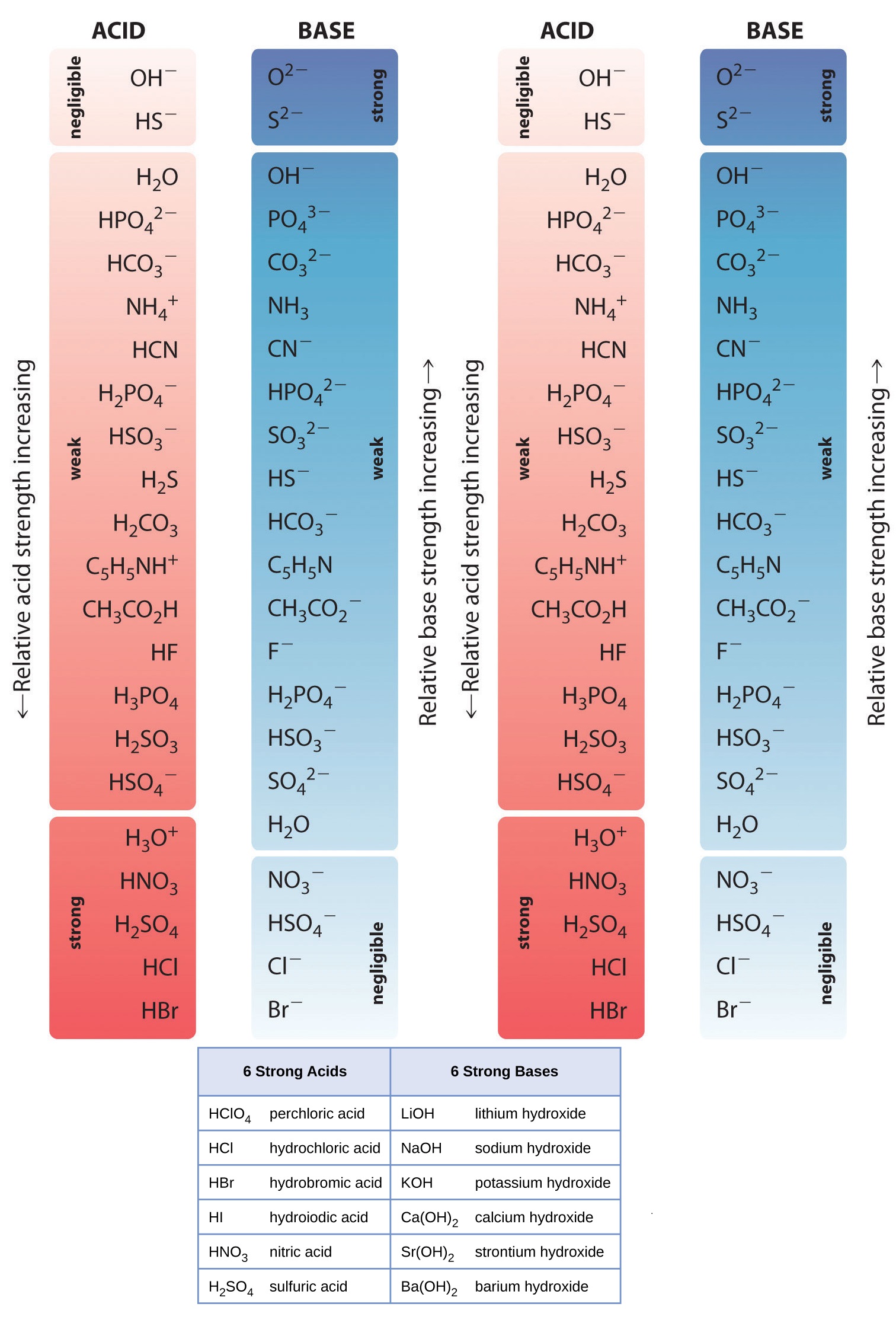
List of Strong Acids & Bases in Order StudyPK
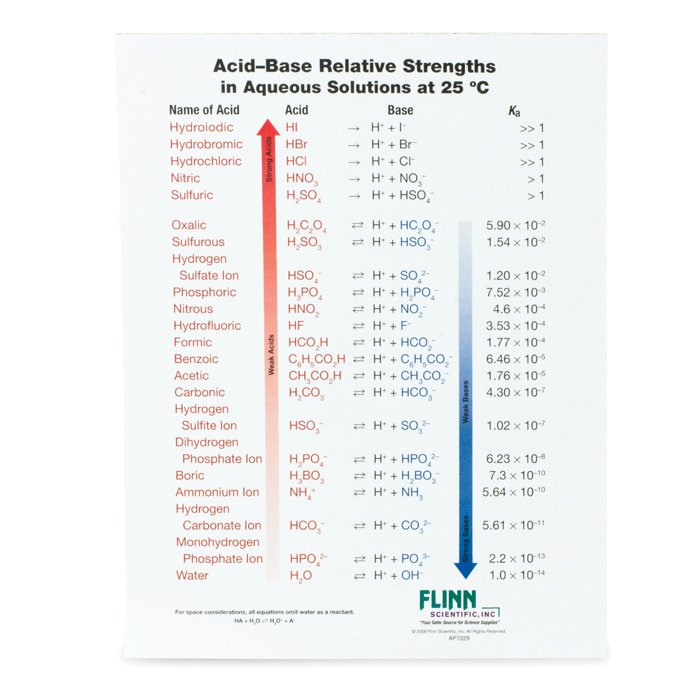
AcidBase Strength Charts for Chemistry
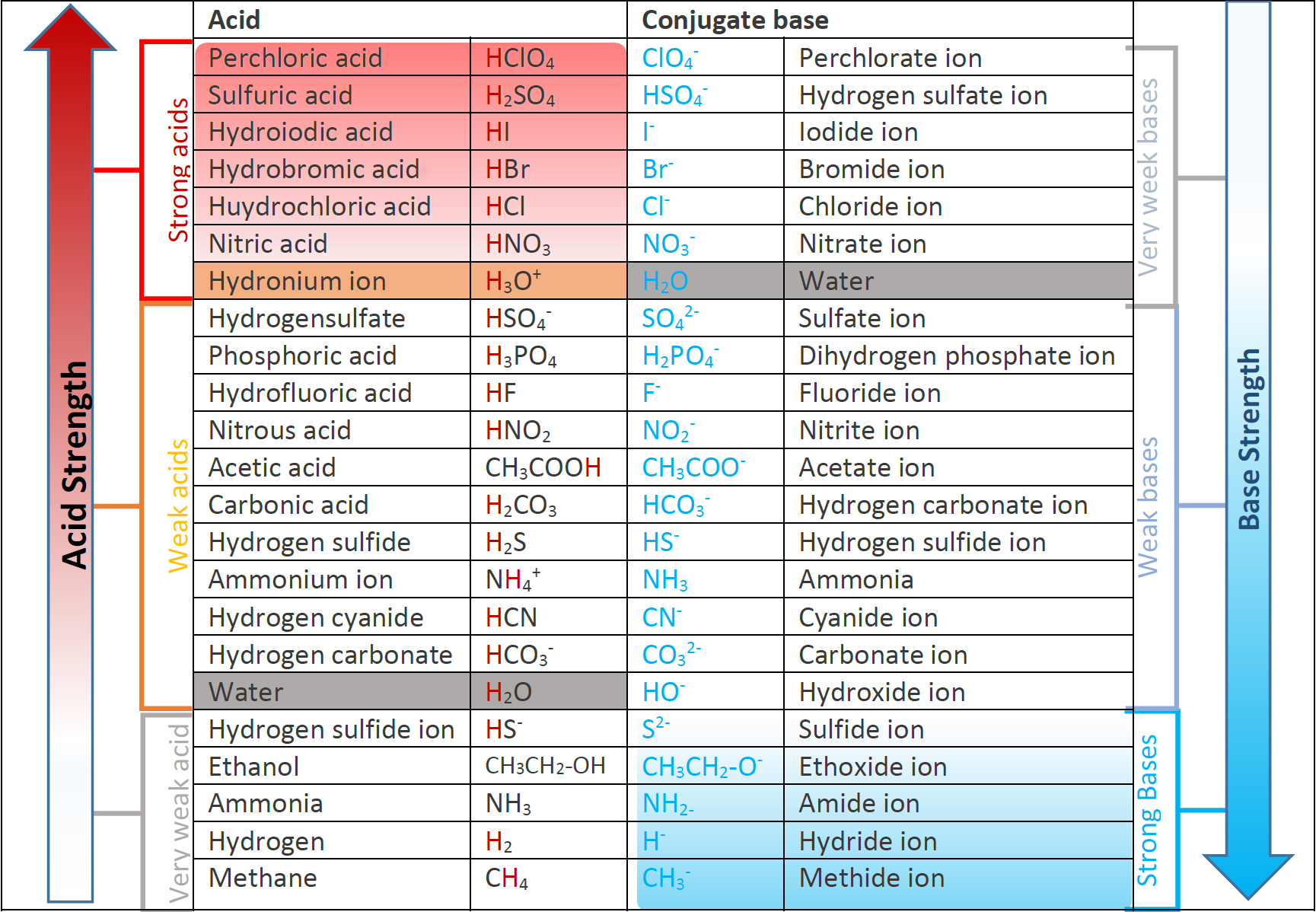
6.3 Strength of acids and bases Chemistry LibreTexts
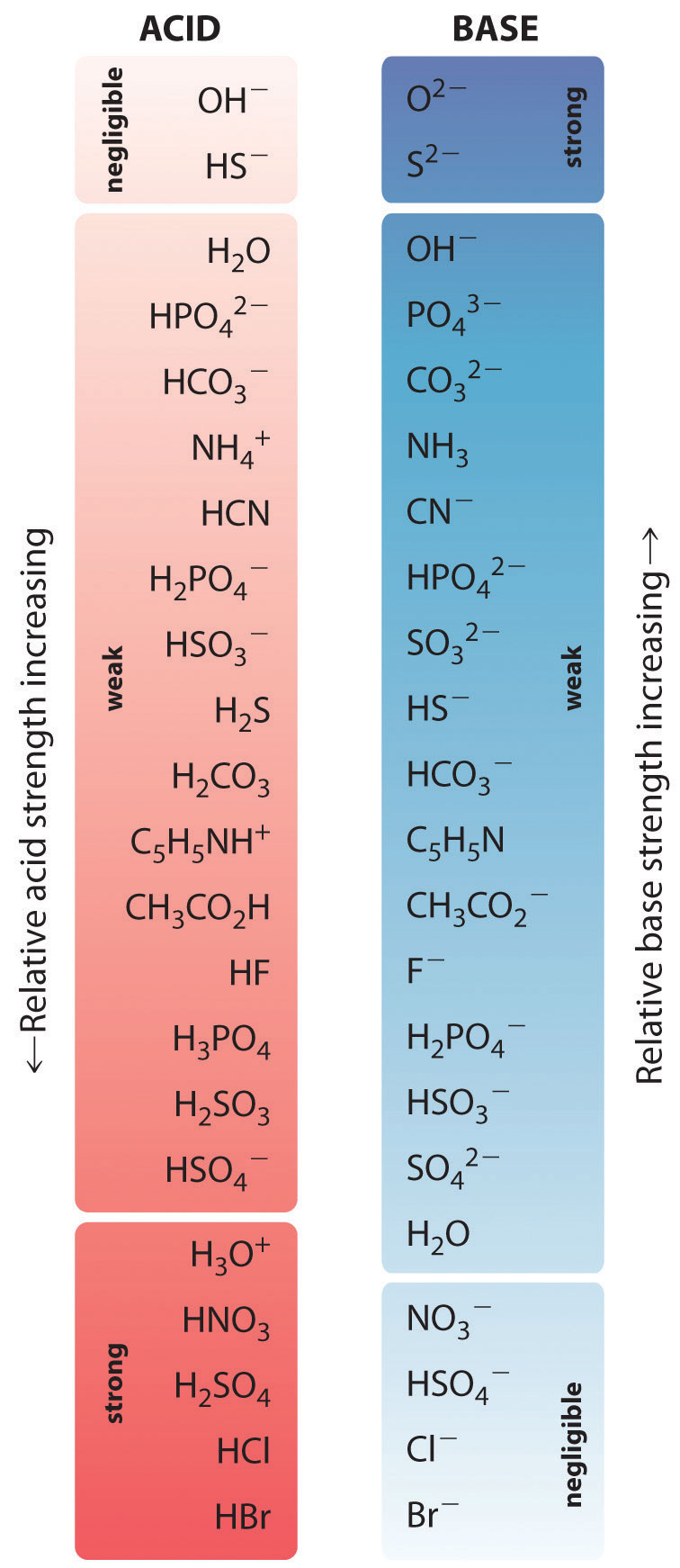
Acid Strengths Table
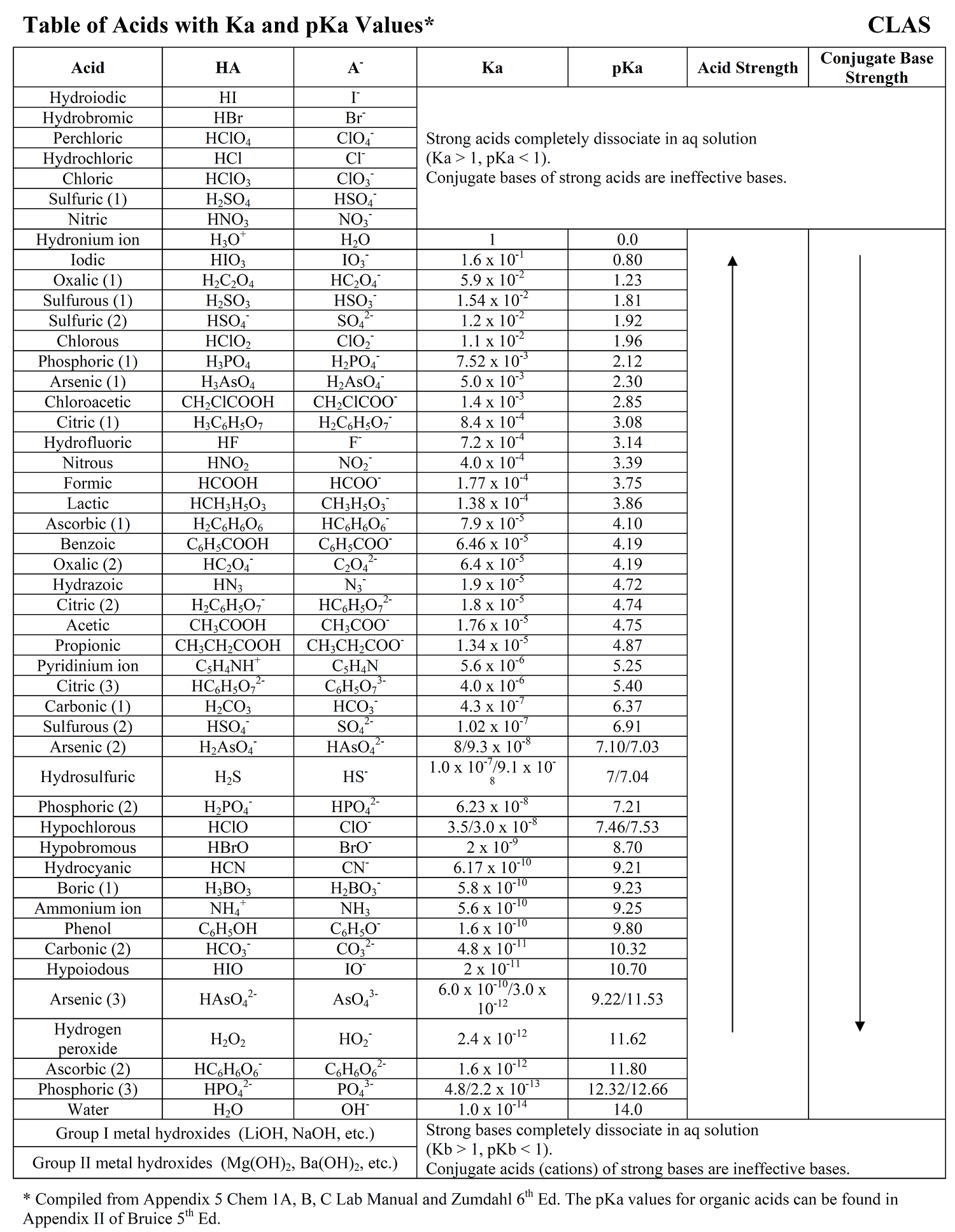
Ka Values And Acids
pKa Values and strengths of Acids and Bases

Acids Facts, Summary, Weak & Strong ALevel Chemistry Revision
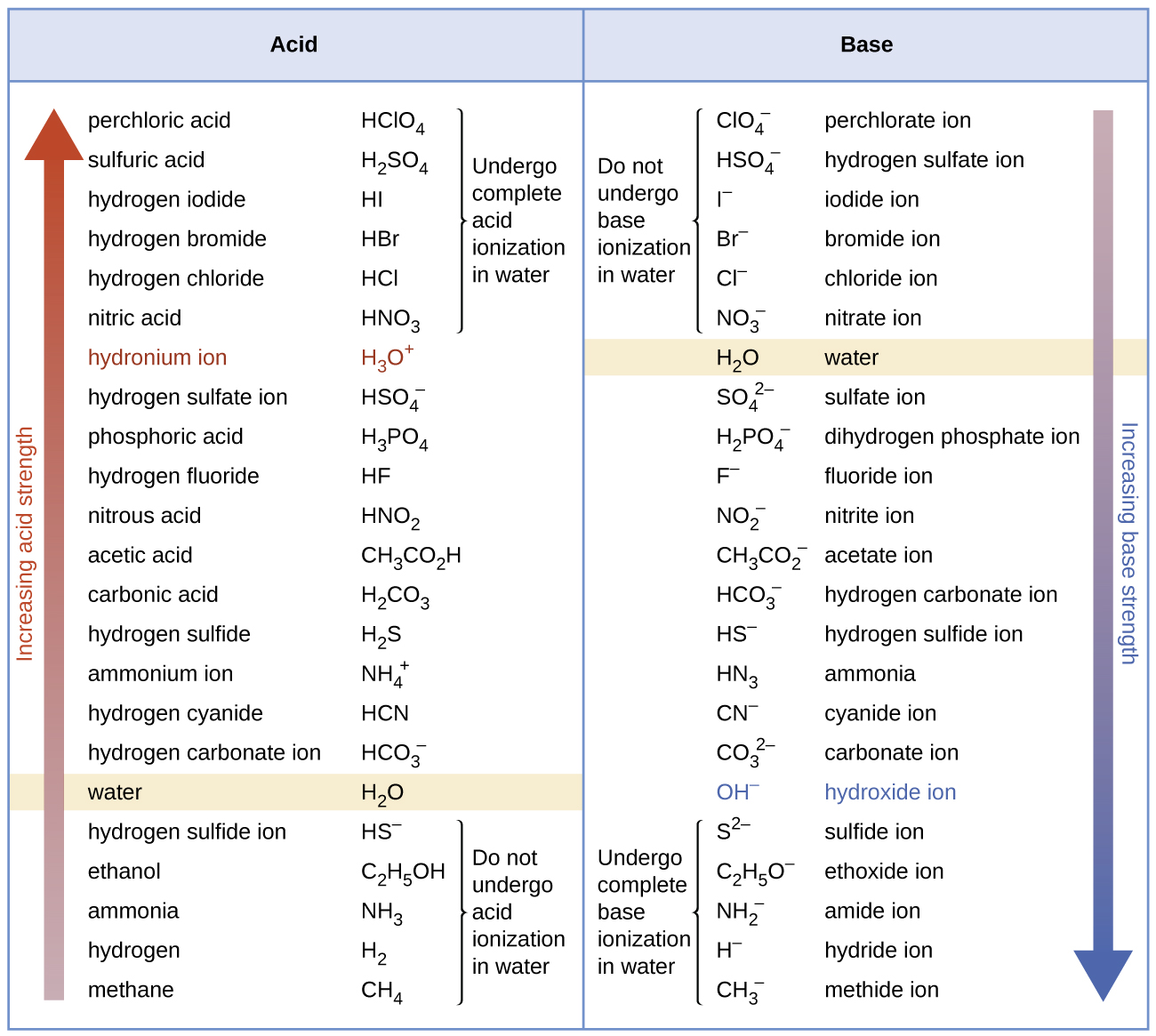
14.3 Relative Strengths of Acids and Bases Chemistry 112 Chapters 12
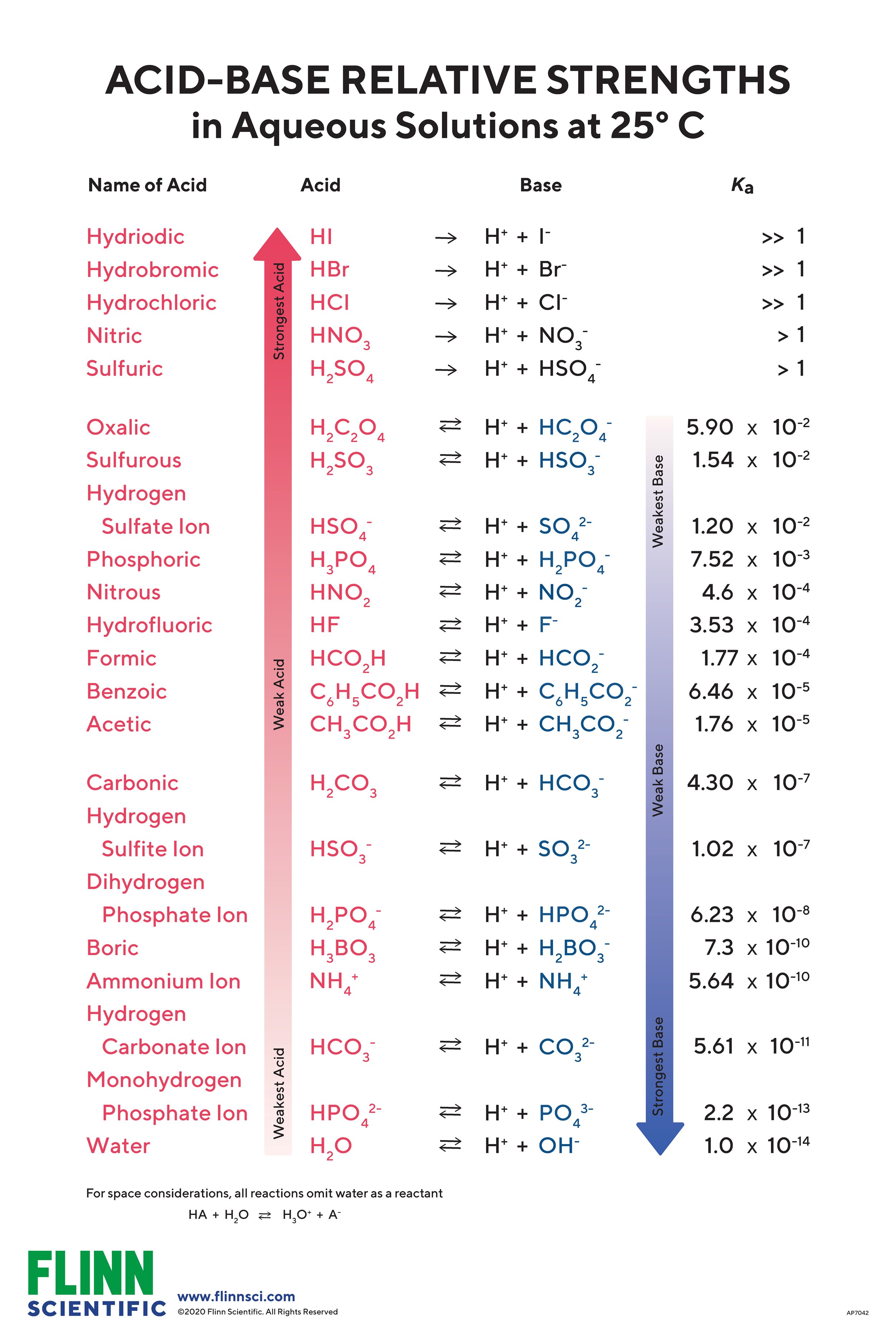
AcidBase Strength Chart
Web Use This Acids And Bases Chart To Find The Relative Strength Of The Most Common Acids And Bases.
Acid Strength Depends On A Variety Of Chemical Factors, Including Electronegativity, Atomic Radius, And Resonance.
Acid Or Base Strength Is A Measure Of How Readily The Molecule Ionizes In Water.
Acid Or Base Strength Is A Measure Of How Readily The Molecule Ionizes In Water.
Related Post:
